16-3 Understanding and Treating Neurological Disorders
577
In everyone’s lifetime, at least one close friend or relative will develop a neurological disorder, even if we ourselves escape them. Disorder causes are understood in a general sense, and for most, rehabilitative treatment is emerging. In this section we review some common neurological disorders: traumatic brain injury, stroke, epilepsy, multiple sclerosis, and neurodegenerative disorders.
Traumatic Brain Injury
Traumatic brain injury (TBI), a wound to the brain that usually results from a blow to the head, is the most common form of brain damage in people under age 40. TBI commonly results from the head making impact with other objects—
Section 14-5 details a dancer’s recovery from TBI.
The two most important factors in the incidence of head trauma are age and sex. Children and elderly people are more likely to injure their head in a fall than are others, and males between 15 and 30 incur brain injury especially in automobile and motorcycle accidents (Figure 16-5). A child’s chance of having significant traumatic brain injury before he or she is old enough to drive is 1 in 30.
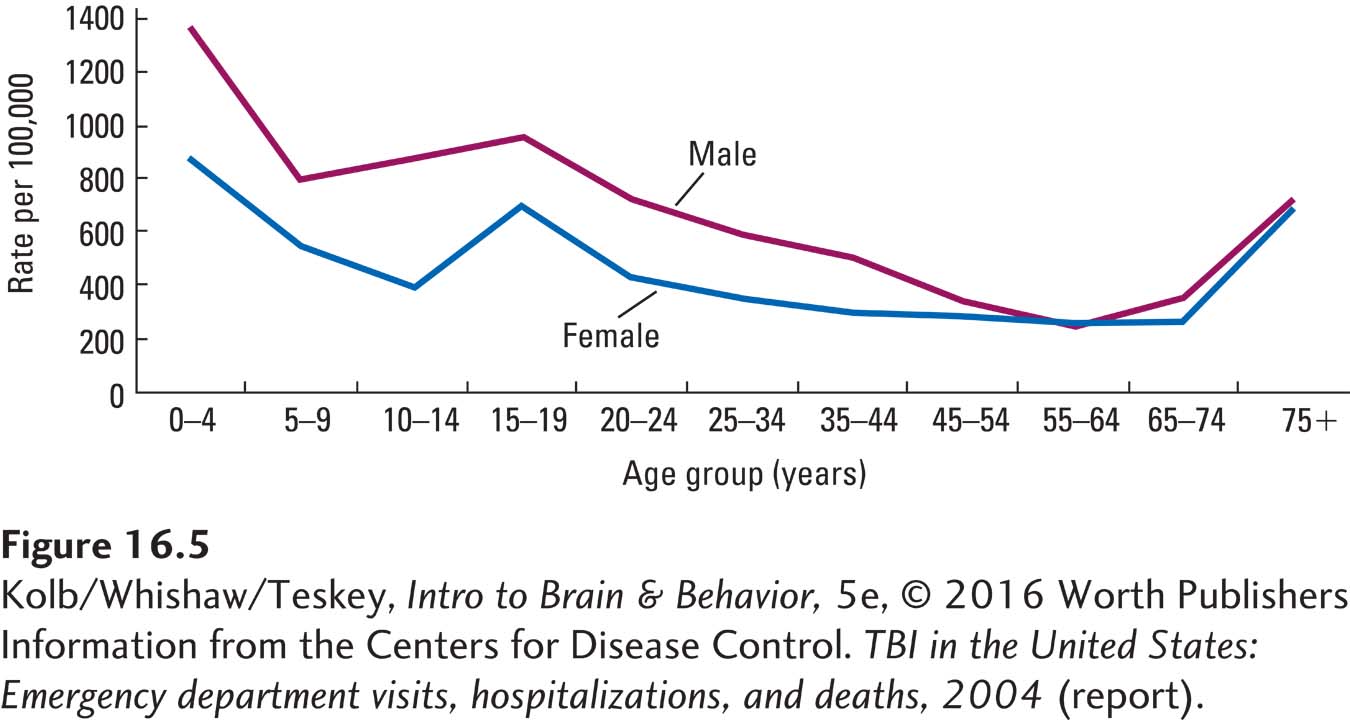
Concussion is a critical concern for both professional and amateur athletes, especially those who play football, ice hockey, lacrosse, soccer, and no less for those on active military duty. Sports account for about 20 percent of TBIs, and the U.S. Army Institute of Surgical Research reports that traumatic brain injury affects more than 1 in 5 U.S. soldiers wounded in war.
In longitudinal research, over time investigators repeatedly observe or examine subjects with respect to the study’s variable(s).
A large-
Symptoms and Outcome of Brain Trauma
TBI can cause direct damage to the brain. Trauma can disrupt the brain’s blood supply, induce bleeding (leading to increased intracranial pressure), cause swelling (leading to increased intracranial pressure), expose the brain to infection, and scar brain tissue (the scar being a focus for later epileptic seizures). The disruption in blood supply tends to be brief, but a parallel disruption of energy production by neuronal mitochondria, which can persist for weeks, is related to many postconcussion behavioral symptoms.
578
CLINICAL FOCUS 16-3
Concussion
Early in 2011, 50-
As a pro player he endured at least 10 concussions, but they did not seem serious enough to cause him to leave the game. After retiring from football, he went to Harvard and obtained a business degree. He pursued a successful business career until he began to have trouble making decisions and controlling his temper.
Eventually, Duerson’s business and marriage failed. After his suicide, the Center for the Study of Traumatic Encephalopathy in Boston did study his brain. The Center is conducting postmortem anatomical analyses of the brains of former athletes as part of a long-
Duerson’s diagnosis, chronic traumatic encephalopathy (CTE), is a progressive degenerative disease found in individuals with a history of multiple concussions and other closed-
Concussion, the common term for mild traumatic brain injury (MTBI), is common in sports, especially contact sports, including American football, ice hockey, and rugby. Concussion also results from falls in many other sports and from vehicular accidents. It is likely that the incidence of concussion is higher than 6 per 1000 individuals.
Concussion often goes unrecognized. For those that are diagnosed, little apparent pathology appears after relatively short periods of rest, the usual treatment. Nevertheless, the relationship is well established between concussion and a range of degenerative diseases that occur later in life—
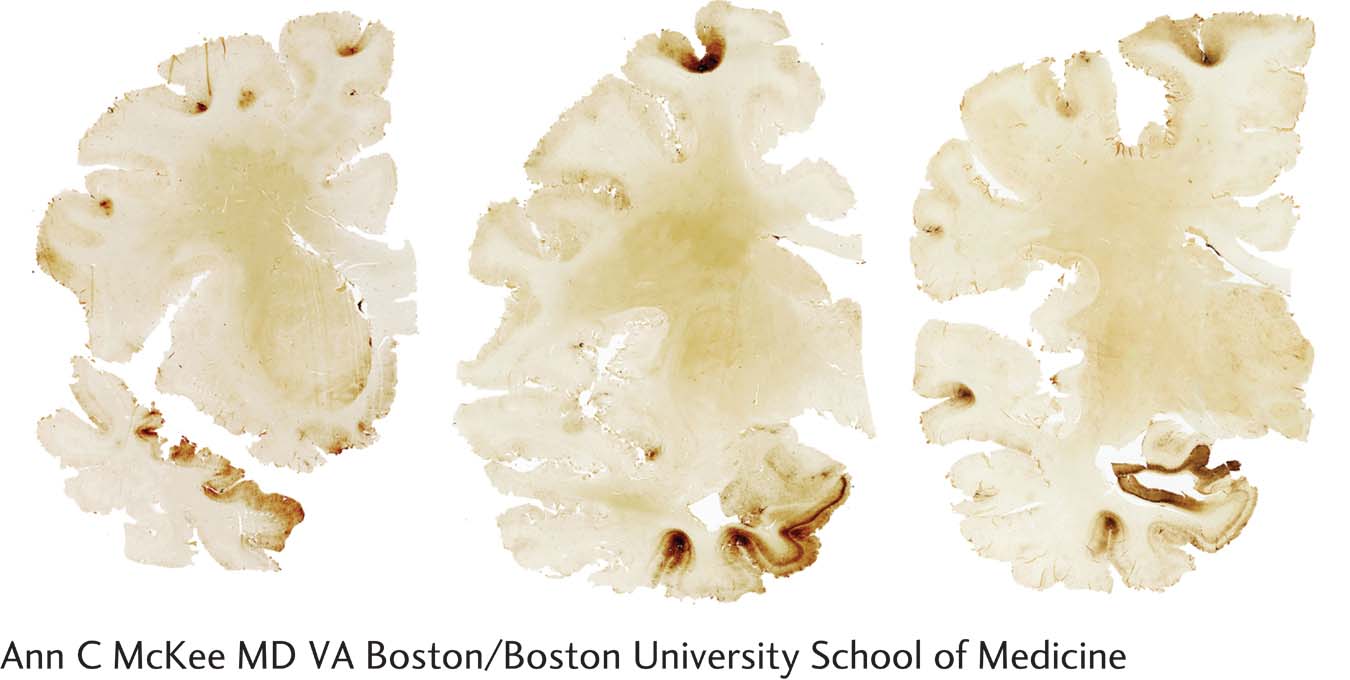
The relationship between concussion in early life and later degenerative brain disease suggests that concussion can initiate a cascade of pathological events that over years develop into CTE. CTE is characterized by neurofibrillary tangles, plaques, and neuronal death. Cerebral atrophy and expanded ventricles due to cell loss are typical in advanced cases. As shown in the illustration, researchers test for cell death by staining for accumulation of the tau protein, which is associated with neuronal death and so is a sensitive marker for brain trauma.
The unknowns about CTE are many. Is just one or are many concussions required to initiate a cascade that results in CTE? Are individuals who get CTE especially susceptible? What constitutes a concussion? Should blows to the head that result in no pronounced symptoms be distinguished from blows that result in loss of consciousness?
What we do know is that many well-
Section 1-2 presents a case study on recovering consciousness following TBI.
Traumatic brain injury is commonly accompanied by a loss of consciousness that may be brief (minutes) or prolonged (coma). Duration of unconsciousness can serve as a measure of the severity of damage, because it correlates directly with mortality, intellectual impairment, and deficits in social skills. The longer coma lasts, the greater the possibility of serious impairment or death.
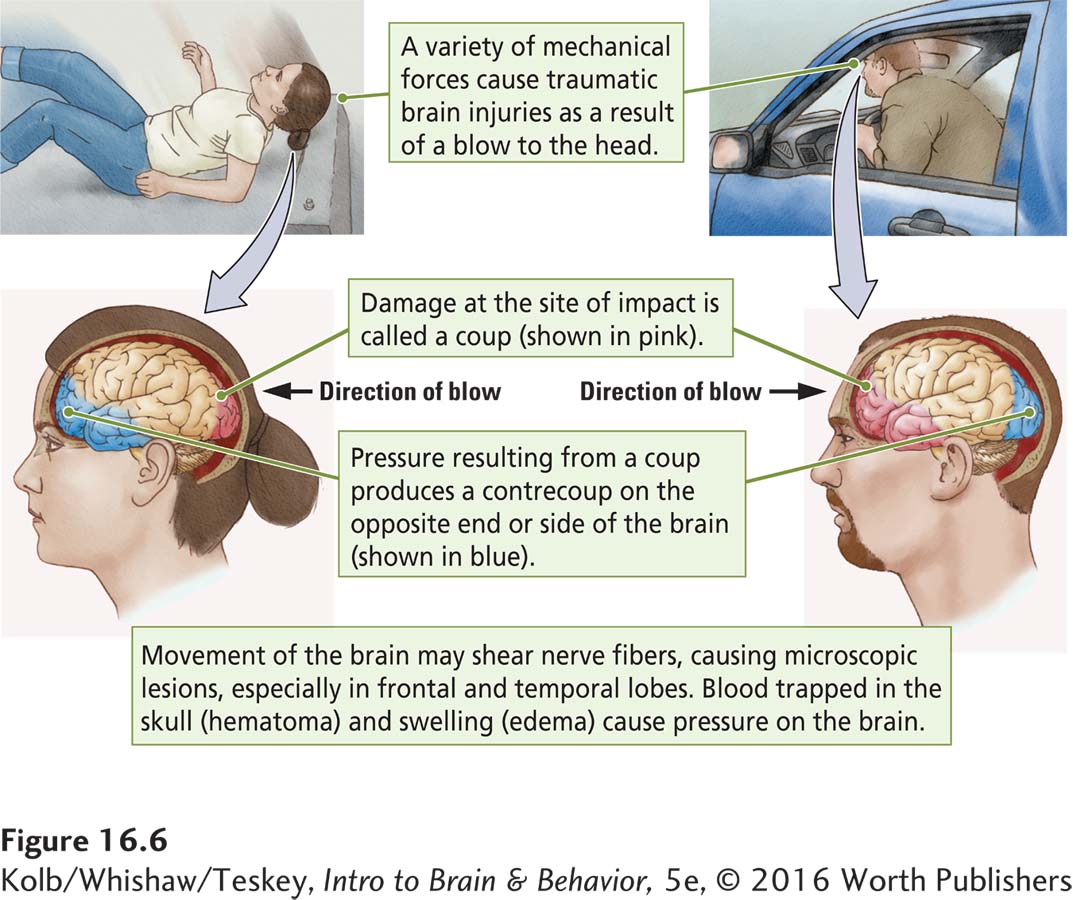
Two kinds of behavioral effects result from TBI: (1) impairment of specific functions mediated by the cortex at the coup (the site of impact) or contrecoup (opposite side) lesion, as illustrated in Figure 16-6, and (2) more generalized impairments from widespread trauma throughout the brain. Discrete impairment is most commonly associated with damage to the frontal and temporal lobes, the brain areas most susceptible to TBI. (See tissue samples in Focus 16-3.)
579
More generalized impairment results from minute lesions and lacerations scattered throughout the brain. Movement of the hemispheres in relation to one another causes tearing characterized by a loss of complex cognitive functions, including mental speed, concentration, and overall cognitive efficiency.
TBI patients generally complain of poor concentration or lack of ability. They fail to do things as well as they could before the injury, even though their intelligence is unimpaired. In fact, in our experience, people with high skill levels seem to be the most affected by TBI, in large part because they are acutely aware of loss of a skill that prevents them from returning to their former competence level.
Traumatic brain injury that damages the frontal and temporal lobes also tends to significantly affect personality and social behavior. Few victims of traffic accidents who have sustained severe head injuries ever resume their studies or return to gainful employment. If they do reenter the work force, they do so at a lower level than before their accident.
One frustrating problem with traumatic brain injury is misdiagnosis: chronic effects of injuries often are unaccompanied by any obvious neurological signs or abnormalities in CT or MRI scans. Patients may therefore be referred for psychiatric or neuropsychological evaluation. MRI-
Section 7-3 introduces the MRS technique.
MRS, a modification of MRI, can identify changes in specific markers of neuronal function. One such marker is N-acetylaspartate (NAA), the second most abundant amino acid in the human brain. Assessing the level of NAA expression provides a measure of neuronal integrity, and deviations from normal levels (up or down) can be taken as a marker of abnormal brain function. People with traumatic brain injury show a chronic decrease in NAA that correlates with the severity of the injury. Although not yet in wide clinical use, MRS is a promising tool, not only for identifying brain abnormalities but also for monitoring cellular response to therapeutic interventions.
Recovery from Traumatic Brain Injury
580
Recovery from head trauma may continue for 2 to 3 years and longer, but most cognitive recovery occurs in the first 6 to 9 months. Recovery of memory functions appears to be slower than recovery of general intelligence, and the final level of memory performance is lower than for other cognitive functions. People with brainstem damage, as inferred from oculomotor disturbance, have a poorer cognitive outcome, and a poorer outcome is probably true of people with initial dysphasias or hemiparesis as well.
Although the prognosis for significant recovery of cognitive functions is good, optimism about the recovery of social skills or personality traits, areas that often show significant change, is less rosy. Findings from numerous studies support the conclusions that quality of life—
Stroke
Focus 2-3 describes the symptoms and aftereffects of stroke.
Diagnosticians may be able to point to a specific immediate cause of stroke, an interruption of blood flow from either blockage of a vessel or bleeding from a vessel. This initial event, however, merely sets off a sequence of damage that progresses, even if the blood flow is restored. Stroke results in a lack of blood, called ischemia, followed by a cascade of cellular events that wreak the real damage. Changes at the cellular level can seriously compromise not only the injured part of the brain but other brain regions as well.
Effects of Stroke
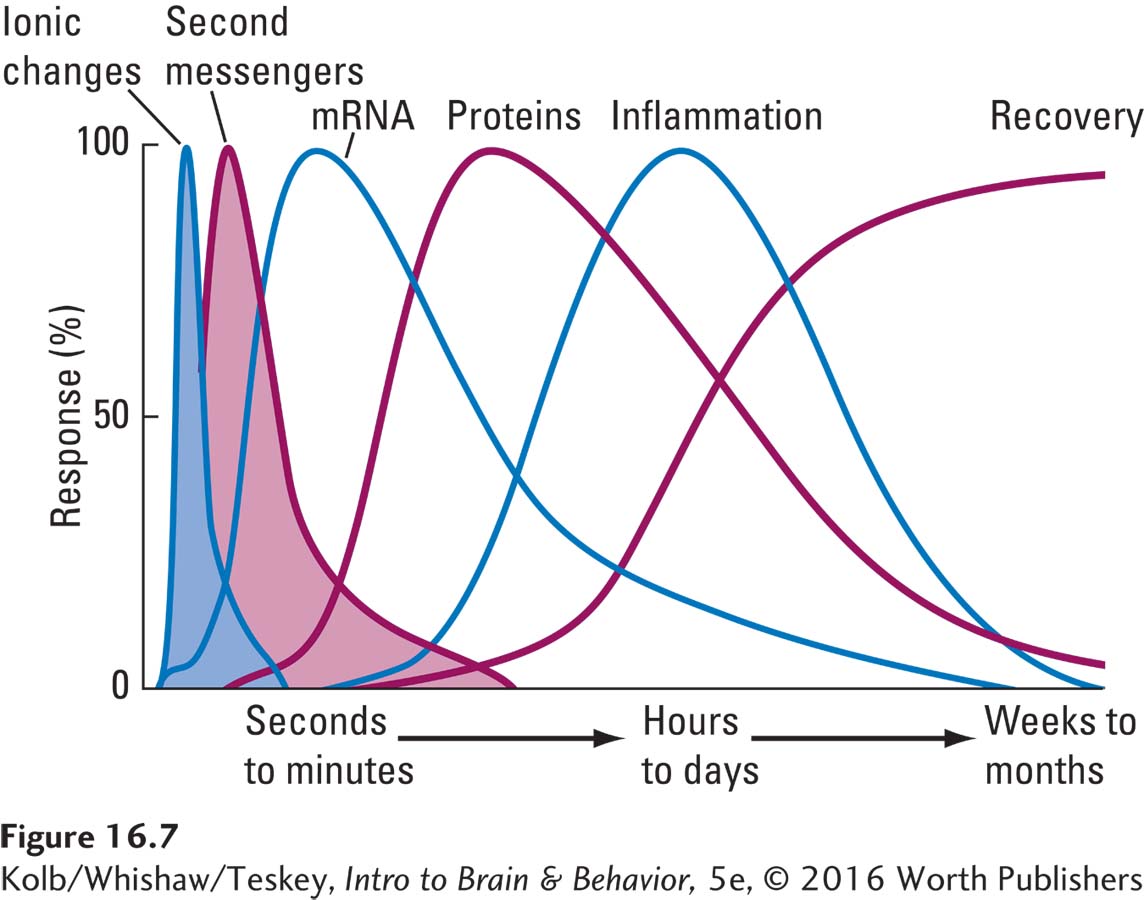
Consider what happens after a stroke interrupts the blood supply to a cerebral artery. In the first seconds to minutes after ischemia, as illustrated in Figure 16-7, changes begin in the affected regions’ ionic balance, including changes in pH and in the properties of the cell membrane. These ionic changes result in several pathological events.
Figure 5-4 shows how calcium affects neurotransmitter release; Figure 5-15, how metabotropic receptors can activate second messengers.
Release of massive amounts of glutamate results in prolonged opening of calcium channels in cell membranes.
Open calcium channels in turn allow toxic levels of calcium to enter the cell, not only producing direct toxic effects but also instigating various second-
messenger pathways that can harm neurons. In the ensuing minutes to hours, mRNA is stimulated, altering protein production in the neurons and possibly proving toxic to the cells. Brain tissues become inflamed and swollen, threatening the integrity of cells that may be far removed from the stroke site. As in TBI, an energy crisis ensues as mitochondria reduce their production of ATP, resulting in less cerebral energy.
A form of neural shock occurs. During this diaschisis, areas distant from the damage are functionally depressed. Thus, not only are local neural tissue and its function lost but areas related to the damaged region also undergo a sudden withdrawal of excitation or inhibition.
Stroke may also be followed by changes in the injured hemisphere’s metabolism, its glucose utilization, or both. These changes may persist for days. As with diaschisis, the metabolic changes can severely affect the functioning of otherwise healthy tissue. For example, after a cortical stroke, metabolic rate has been shown to decrease about 25 percent throughout the hemisphere.
Treatments for Stroke
The ideal treatment is to restore blood flow in blocked vessels before the cascade of nasty events begins. One clot-
| Use the F.A.S.T. Test to Spot Stroke |
|---|
| Face Ask for a smile to check both understanding and muscle control. |
| Arms Check if one arm is weak by asking the person to raise both arms. |
| Speech Listen for slurred speech. |
| Time If you see any symptom, call 911 or the local emergency services number right away. |
581
Other drugs called neuroprotectants can be used to try to block the cascade of postinjury events, but to date we have no truly effective drugs. Clinical trials based on animal studies have generally failed, in part because understanding what the appropriate brain targets should be is limited.
When the course of the stroke leads to dead brain tissue, the only treatments that can be beneficial are those that facilitate plastic changes in the remaining brain. Examples are speech therapy and physical therapy. Revolutionary approaches to stroke rehabilitation use virtual reality, computer games, and robotic machines (Laver et al., 2015).
Experiment 11-3 describes research with monkeys that contributed to developing constraint-
Still, some simple treatments are surprisingly effective. One is constraint-
In constraint-
Another common effect of stroke is loss of speech. Specific speech therapy programs can aid in the recovery of speech. Music and singing, mediated in part by the right hemisphere, can augment speech therapy after left hemisphere stroke.
Therapies using pharmacological interventions (e.g., noradrenergic, dopaminergic, cholinergic agonists) combined with behavioral therapies provide equivocal gains in stroke patients. The bulk of evidence suggests that patients with small gray matter strokes are most likely to show benefits from these treatments, whereas those with large strokes that include white matter show little benefit.
Finally, there have been many attempts to use either direct cortical stimulation or TMS in combination with behavioral therapy as a stroke treatment. The idea is to induce plasticity in regions adjacent to the dead tissue with the goal of enhancing the efficiency of the residual parts of the neuronal networks. These treatments have proved beneficial in patients with good residual motor control, but again, those with larger injuries show much less benefit, presumably because the residual neuronal network is insufficient.
Epilepsy
Focus 4-1 describes a diagnosis of epilepsy and shows an EEG being recorded.
Epilepsy is characterized by recurrent seizures, which register on an electroencephalogram (EEG) as highly synchronized neuronal firing indicated by a variety of abnormal waves. About 1 person in 20 has at least one seizure in his or her lifetime, usually associated with an infection, temperature, and hyperventilation during childhood (6 months to 5 years of age). Most children who experience a seizure do not develop epilepsy, which affects between 0.2 percent and 4.1 percent of the population. Developed nations record a lower prevalence and incidence of epilepsy compared with developing nations.
Classifying Seizures
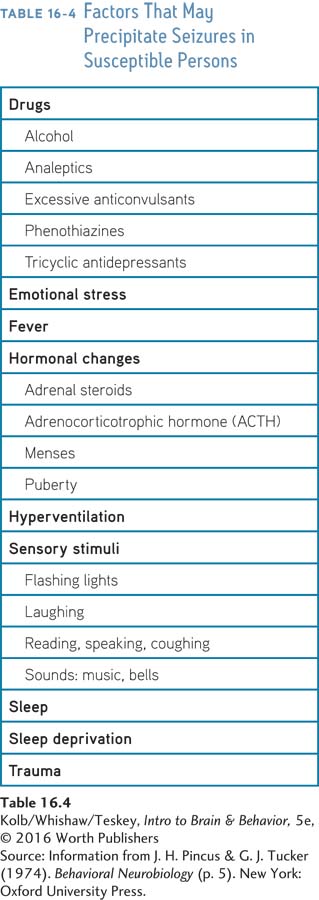
Causes of epileptic seizures are categorized as genetic, structural/metabolic, or unknown. Genetic epilepsy results directly from a known genetic defect. Causes of structural/metabolic epilepsy include brain malformations and tumors, acquired disorders such as stroke and trauma, and infections. The unknown category encompasses causes yet to be identified. Table 16-4 summarizes the great variety of circumstances that appear to precipitate a seizure. The range of circumstances is striking, but seizures do have a consistent feature: they are most likely to occur when a person is sleeping.
582
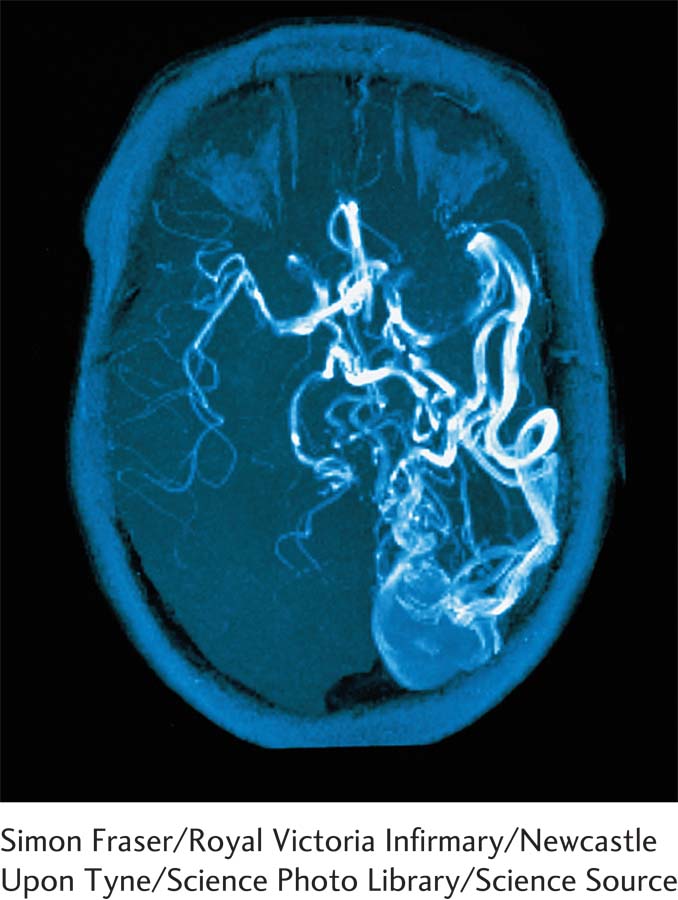
Seizures are classified by the way participating neural networks are distributed. Focal seizures arise from a synchronous, hyperactive local brain region. Thus, focal seizures may have motor, sensory, autonomic, and/or psychogenic features. Focal seizures are further classified as to whether awareness is retained or altered (dyscognitive seizure). Generalized seizures may start at a focal location, then spread rapidly and bilaterally to distributed networks in both hemispheres. In primary generalized epilepsy, seizures begin in more widespread neural networks. Seizure diagnosis is improved by the use of a wearable device that records them (Gubbi et al., 2015).
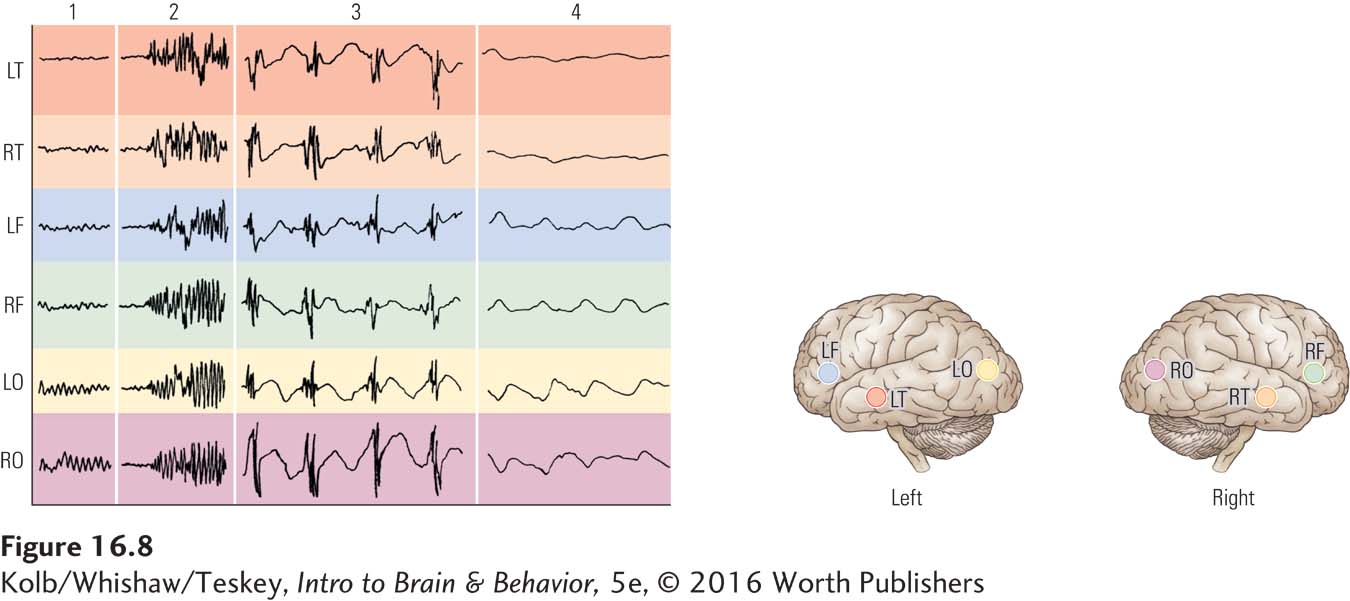
People having a seizure may cycle through the four stages charted in Figure 16-8: (1) normal EEG record before onset; (2) onset and tonic phase, in which the body stiffens; (3) clonic phase, in which the person makes rhythmic movements in time with the large, highly synchronized discharges; and (4) a period of depressed EEG activity after the seizure ends.
For the most part, seizures self-
People with epilepsy die unexpectedly at a rate 24 times that of the general population. Sudden unexpected death in epilepsy (SUDEP) has no identifiable cause (such as status epilepticus, drowning, or falling). Most evidence suggests that SUDEP occurs during or more often after a seizure. Respiratory and cardiac failure may be common causes, but events leading up to SUDEP have yet to be characterized.
Treating Epilepsy
The first-
Multiple Sclerosis
583
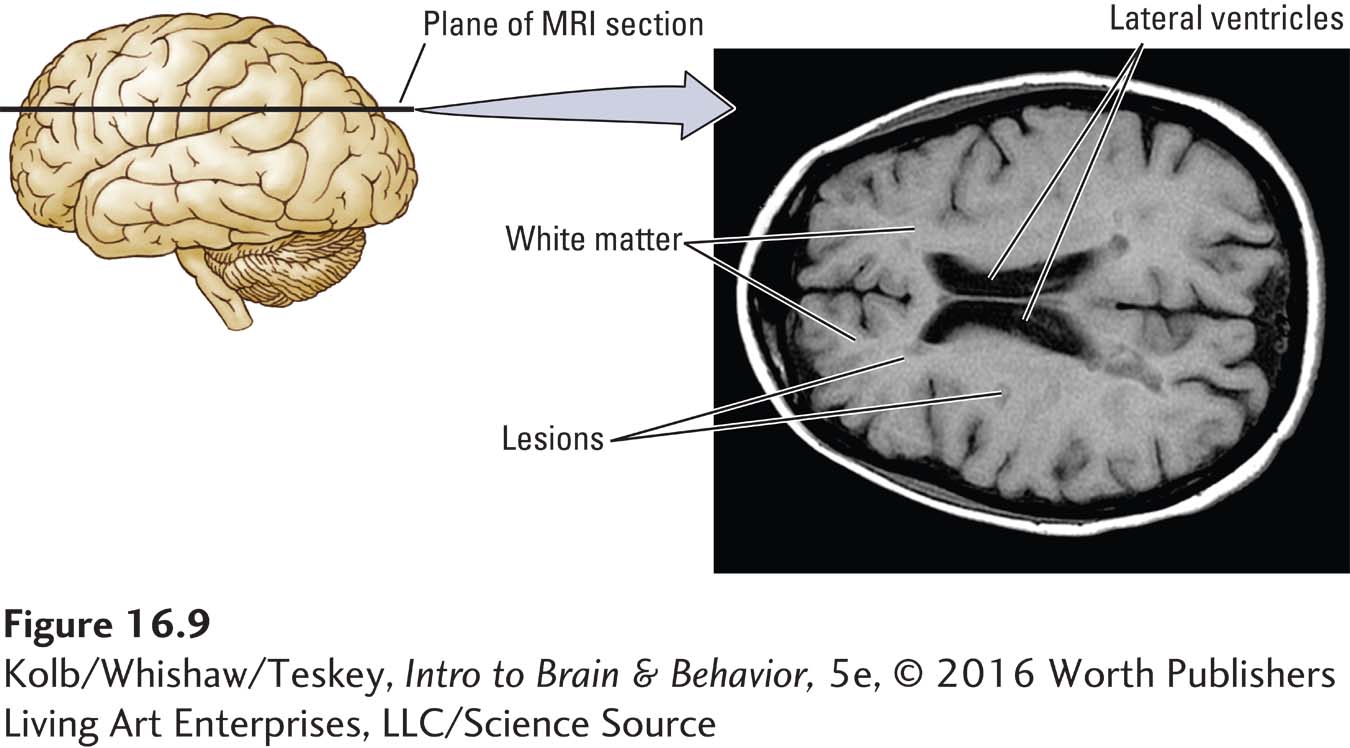
In multiple sclerosis (MS), the myelin that encases axons is damaged and neuronal functions are disrupted. MS is characterized by myelin loss in both motor and sensory tracts and nerves. The oligodendroglia that form the myelin sheath, and in some cases the axons, are destroyed. Brain imaging with MRI, as shown in Figure 16-9, identifies areas of sclerosis in the brain as well as in the spinal cord.
Remission followed by relapse is a striking feature of MS: in many cases, early symptoms initially are followed by improvement. The course varies, running from a few years to as long as 50 years. Paraplegia, the classic feature of MS, may eventually confine the affected person to bed.
Worldwide, about 1 million people have MS; women outnumber men about two to one. Multiple sclerosis is most prevalent in northern Europe and northern North America, rare in Japan and in more southerly or tropical countries. Depending on region, incidence of MS ranges from 2 to 150 per 100,000 people, making it one of the most common structural nervous system diseases.
The cause or causes of MS are unknown. Proposed causes include bacterial infection, a virus, environmental factors including pesticides, an immune response of the central nervous system, misfolded proteins, and lack of vitamin D. Often, multiple cases occur in a single family. Many genes have been associated with MS, but no clear evidence indicates as yet that MS is inherited or transmitted from one person to another.
584
Section 4-4 describes another autoimmune disease, myasthenia gravis.
Research has focused on the immune system’s relation to MS and the possibility that MS is an autoimmune disease. The ability to discriminate between a foreign pathogen in the body and the body itself is central to immune system functioning. If this discrimination fails, the immune system makes antibodies to a person’s own body, in this case, to myelin.
As various organisms’ genomes have been sequenced, it has become apparent that all have many genes in common. Thus the proteins found in different organisms are surprisingly similar. And here is the problem for the human immune system: a foreign microbe may contain proteins that are nearly identical to the body’s own proteins. If microbe and human have a common gene sequence, the immune system can mistakenly attack itself, a process known as horror autotoxicus.
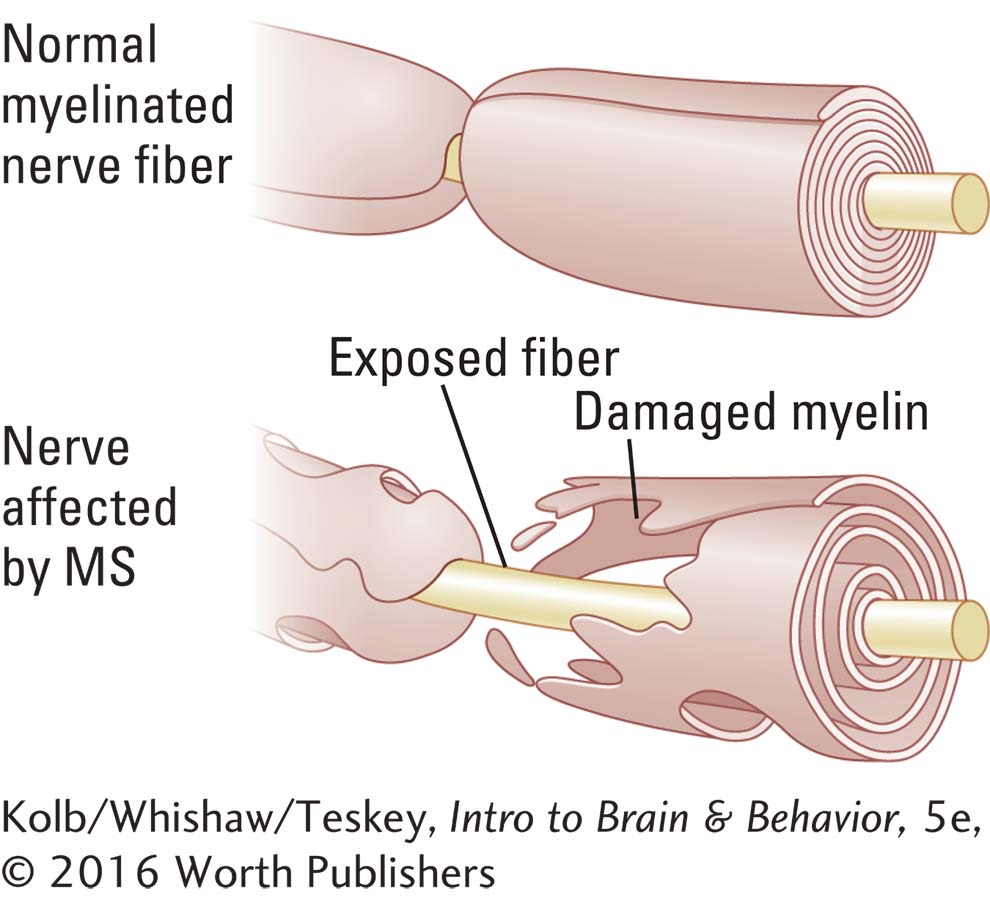
Many microbial protein sequences are homologous with structures found in myelin, which leads to an attack against the microbe and a person’s own myelin. Research showing the important role of the immune system in MS has intensified work on developing new treatments (Kornek, 2015). One strategy is to build up immune system tolerance by injecting DNA-
That MS is more common in extreme northern and southern latitudes has raised the possibility that inadequate direct sunlight, which is necessary for the body to synthesize vitamin D, factors into precipitating the condition (Sundström & Salzer, 2015). Lack of understanding whether an acute or a longstanding deficiency is relevant complicates identifying a possible relationship. Possibly a vitamin D deficiency interacts with other factors to increase susceptibility to MS. For example, a number of genes are involved in the transport and absorption of vitamin D and variants of these genes are related to low levels of vitamin D.
Some research has suggested that MS might originate from insufficient blood drainage from the brain, which can be improved by cleaning or expanding veins from the brain, including the jugular, to improve drainage. The treatment has been called liberation therapy for its potential relief from a condition of chronic cerebrospinal venous insufficiency (CCSVI). Internet discussion worldwide has led to Web sites advertising treatment using vascular surgery for patients with MS. Numerous studies are examining a plethora of questions related to CCSVI. Primary among them: Is venous flow restricted in people with MS? Optimism about an outcome has far outpaced the science, which questions the treatment (Tsivgoulis et al., 2015).
Neurodegenerative Disorders
Human societies have never before undergone the age-
Dementias affect 1 percent to 6 percent of the population older than age 65 and 10 percent to 20 percent of those older than age 80. For every person diagnosed with dementia, it is estimated that several others endure undiagnosed cognitive impairments that affect their quality of life. Currently, more than 6 million people in the United States have a dementia diagnosis, a number projected to rise to about 15 million by 2050. By then, 1 million new U.S. cases per year will be emerging. Extending these projections across the rest of the developed world portends staggering social and economic costs (Khachaturian & Khachaturian, 2015). The World Health Organization estimates that by 2050, the incidence of dementia will balloon to 135.5 million people worldwide.
Types of Dementia
585
Dementia is an acquired and persistent syndrome of intellectual impairment. Its two essential features are (1) loss of memory and other cognitive deficits and (2) impairment in social and occupational functioning. Dementia is not a singular disorder, but there is no clear agreement on how to split up subtypes. Daniel Kaufer and Steven DeKosky (1999) divide dementias into the broad categories of degenerative and nondegenerative (Table 16-5).
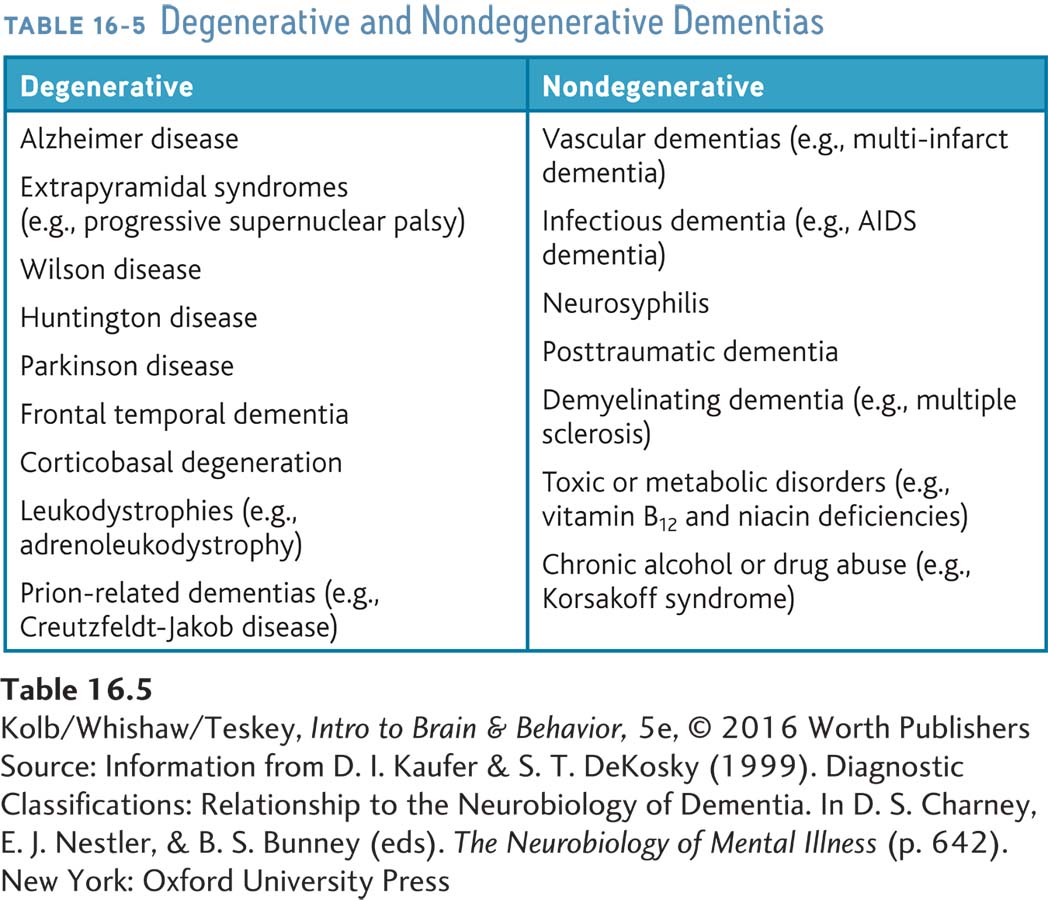
Nondegenerative dementias, a heterogeneous group of disorders with diverse causes, including diseases of the vascular or endocrine systems, inflammation, nutritional deficiency, and toxins, are summarized on the right in Table 16-5. The most prevalent cause is vascular. The most significant risk factors for nondegenerative dementias are chronic hypertension, obesity, sedentary lifestyle, smoking, and diabetes. All are as well risk factors for cardiovascular disease. Degenerative dementias, listed on the left in the table, presumably have a degree of genetic transmission.
We now review two degenerative dementias, Parkinson and Alzheimer diseases. Both pathological processes are primarily intrinsic to the nervous system, and both tend to affect certain neural systems selectively.
Parkinson Disease
Figure 7-5 diagrams degeneration in the substantia nigra associated with Parkinson symptoms.
Parkinson disease is common. Estimates of its incidence vary up to 1.0 percent of the population, rise sharply in old age, and are certain to grow in coming decades. The disease seems related to degeneration of the substantia nigra and attendant loss of the neurotransmitter dopamine produced there and released in the striatum. The disease therefore offers insight into the roles played by the substantia nigra and dopamine in movement control.
That Parkinson symptoms vary enormously illustrates the complexity inherent in understanding a neurological disorder. A well-
Symptoms begin insidiously, often with a tremor in one hand and slight stiffness in distal parts of the limbs. Movements may become slower, the face becoming masklike with loss of eye blinking and poverty of emotional expression. Thereafter the body may stoop and the gait become a shuffle, with arms hanging motionless at the sides. Speech may slow and become monotonous, and difficulty swallowing may cause drooling.
Although the disease is progressive, the rate at which symptoms worsen varies; only rarely is progression so rapid that a person becomes disabled within 5 years. Usually 10 to 20 years elapse before symptoms cause incapacity. A distinctive aspect of Parkinson disease is its on-
Sacks, whose writings enriched the neurological literature beyond measure, died in 2015.
Partial remission may also occur in response to interesting or stimulating situations. Neurologist Oliver Sacks (1998) recounted an incident in which a stationary Parkinson patient leaped from his wheelchair at the seaside and rushed into the breakers to save a drowning man, only to fall back into his chair immediately afterward and become inactive again. Remission of some symptoms in activating situations is common but usually not as dramatic as this case. Simply listening to familiar music can help an otherwise inactive patient get up and dance, for example. Or a patient who has difficulty walking may ride a bicycle or skate effortlessly. Such activities can be used as physical therapy, and physical therapy is important because it may slow disease progression.
586
Positive symptoms are behaviors not typically seen in people. Negative symptoms are the absence of typical behaviors or inability to engage in an activity.
The four major symptoms of Parkinson disease are tremor, rigidity, loss of spontaneous movement (hypokinesia), and postural disturbances. Each symptom may manifest in different body parts in different combinations. Because some symptoms entail the appearance of abnormal behaviors (positive symptoms) and others the loss of normal behaviors (negative symptoms), we consider both major categories.
POSITIVE SYMPTOMS Because positive symptoms are common in Parkinson disease, they are thought to be inhibited, or held in check, in unaffected people but released from inhibition in the process of the disease. Following are the three most common:
Tremor at rest. Alternating movements of the limbs occur when they are at rest and stop during voluntary movements or sleep. Hand tremors often have a pill-
rolling quality, as if a pill were being rolled between the thumb and forefinger. Muscular rigidity. Increased muscle tone simultaneously in both extensor and flexor muscles is particularly evident when the limbs are moved passively at a joint. Movement is resisted, but with sufficient force the muscles yield for a short distance then resist movement again. Thus, complete passive flexion or extension of a joint occurs in a series of steps, giving rise to the term cogwheel rigidity. Rigidity may be severe enough to make all movements difficult—
like moving in slow motion and being unable to speed up the process. Involuntary movements. Small movements or changes in posture, sometimes referred to as akathesia, or cruel restlessness, may accompany general inactivity to relieve tremor and sometimes to relieve stiffness but often occurs for no apparent reason. Other involuntary movements are distortions of posture, such as occur during oculogyric crisis (involuntary turns of the head and eyes to one side), which last minutes to hours.
NEGATIVE SYMPTOMS After detailed analysis of negative symptoms, Jean Prudin Martin (1967) divided patients severely affected with Parkinson disease into five groups:
Disorders of posture. A disorder of fixation presents as an inability or difficulty in maintaining a part of the body in its normal position in relation to other parts. A person’s head may droop forward or a standing person may gradually bend forward, ending up on the knees. Disorders of equilibrium. These disorders cause difficulties in standing or even sitting unsupported. In less severe cases, people may have difficulty standing on one leg, or if pushed lightly on the shoulders, they may fall passively without taking corrective steps or attempting to catch themselves.
Disorders of righting. A person in a supine position has difficulty standing. Many advanced patients have difficulty in even rolling over.
Disorders of locomotion. Normal locomotion requires support of the body against gravity, stepping, balancing while the weight of the body is transferred from one leg to the other, and pushing forward. Parkinson patients have difficulty initiating stepping. When they do walk, they shuffle with short footsteps on a fairly wide base of support because they have trouble maintaining equilibrium when shifting weight from one leg to the other. On beginning to walk, Parkinson patients often demonstrate festination: they take faster and faster steps and end up running forward.
Speech disturbances. One symptom most noticeable to relatives is the almost complete absence of prosody (rhythm and pitch) in the speaker’s voice.
Hypokinesia. Poverty or slowness of movement may also manifest itself in a blankness of facial expression, a lack of blinking or of swinging the arms when walking, a lack of spontaneous speech, or an absence of normal fidgeting. Akinesia also manifests in difficulty making repetitive movements, such as tapping, even in the absence of rigidity. People who sit motionless for hours show hypokinesia in its most striking manifestation.
587
COGNITIVE SYMPTOMS Although Parkinson disease is usually viewed as a motor disorder, changes in cognition occur as well. Psychological symptoms in Parkinson patients are as variable as the motor symptoms. Nonetheless, a significant percentage of patients show cognitive symptoms that mirror their motor symptoms.
Cognitive slowing in Parkinson patients has some parallels to Alzheimer disease.
Oliver Sacks (1998) reported impoverishment of feeling, libido, motive, and attention: people may sit for hours, apparently lacking the will to begin or continue any activity. Thinking seems generally to be slowed and is easily confused with dementia because patients do not appear to be processing the content of conversations. In fact, they may be simply processing very slowly.
Actor Michael J. Fox, a native of Canada pictured in Focus 5-2, was diagnosed with young-
CAUSES OF PARKINSONISM The ultimate cause of Parkinson disease—
TREATING PARKINSON DISEASE The cure for Parkinson disease is either to stop degeneration in the substantia nigra or to replace it. Neither goal is achievable at present. Thus, current treatment is pharmacological and directed toward support and comfort.

Psychological factors influence Parkinsonism’s major symptoms: outcome is affected by how well a person copes. Patients should seek behaviorally oriented treatment early—
The prime objective of pharmacological treatment is increasing the activity in whatever dopamine synapses remain. l-Dopa, a precursor of dopamine, is converted into dopamine in the brain and enhances effective dopamine transmission, as do drugs such as amantadine, amphetamine, monoamine oxidase inhibitors, and tricyclic antidepressants. Anticholinergic drugs, such as atropine, scopolamine, benztropine (Cogentin), and trihexyphenidyl (Artane), block the brain cholinergic systems that seem to show heightened activity in the absence of adequate dopamine activity. As the disease progresses, drug therapies become less effective, and the incidence of side effects increases. Some drug treatments that directly stimulate dopamine receptors have been reported to result in increased sexuality and an increased incidence of compulsive gambling.
Figure 11-13 charts how the GPi, a structure in the basal ganglia, regulates movement force.
Two surgical treatments described in Section 16-2 are based on the idea that increased activity of globus pallidus neurons inhibits motor function. A lesion of the internal part of the globus pallidus (GPi) can reduce rigidity and tremor. Hyperactivity of GPi neurons can also be reduced neurosurgically by electrically stimulating the neurons via deep brain stimulation (see Figure 16-4). A stimulating electrode is permanently implanted in the GPi or an adjacent area, the subthalamic nucleus. Patients carry a small electric stimulator that they can turn on to induce DBS and so reduce rigidity and tremor. These two treatments may be used sequentially: when DBS becomes less effective as the disease progresses, a GPi lesion may be induced.
A promising prospective Parkinson treatment involves increasing the population of dopamine-
588
All these treatments are experimental (Politis & Lindvall, 2012). Before cell replacement will become a useful therapy, many questions must be resolved, including which cell source is best, where in the brain to put grafts, and how new cells can be integrated into existing brain circuits. Stem cells are not a quick fix for Parkinson disease, but the pioneering work on this disease will be instrumental in applying such technology to other diseases.
Anatomical Correlates of Alzheimer Disease
Given the increasing population of elderly people and thus of Alzheimer disease, which accounts for about 65 percent of all dementias, research is directed toward potential causes. Personal lifestyle, environmental toxins, high levels of trace elements such as aluminum in the blood, an autoimmune response, a slow-
Incidence of Alzheimer disease is high in some families, making genetic causes pertinent to understanding disease progression. Risk factors include the presence of the Apoe4 gene, below-
A decade ago, the only way to identify and study Alzheimer disease was postmortem pathology examination. This approach was less than ideal because determining which brain changes came early in the disease and which resulted from those early changes was impossible. Nonetheless, it became clear that widespread changes take place in neocortex and allocortex and that associated changes take place in many neurotransmitter systems. Most of the brainstem, cerebellum, and spinal cord are relatively spared from Alzheimer’s major ravages.
Focus 14-3, on Alzheimer etiology, includes a micrograph of an amyloid plaque.
The principal neuroanatomical change in Alzheimer disease is the emergence of amyloid plaques (clumps of protein from dead neurons and astrocytes), chiefly in allocortex and neocortex. Increased plaque concentration in the cortex has been correlated with the magnitude of cognitive deterioration. Plaques are generally considered nonspecific phenomena in that they can be found in non-
Neurofilaments are a type of tubule that reinforces cell structure, aids its movement, and transports proteins.
Another anatomical correlate of Alzheimer disease is neurofibrillary tangles (accumulations of microtubules from dead cells) found in both neocortex and allocortex, where the posterior half of the hippocampus is affected more severely than the anterior half. Neurofibrillary tangles have been described mainly in human tissue and have also been observed in patients with Down syndrome and Parkinson disease and other dementias.
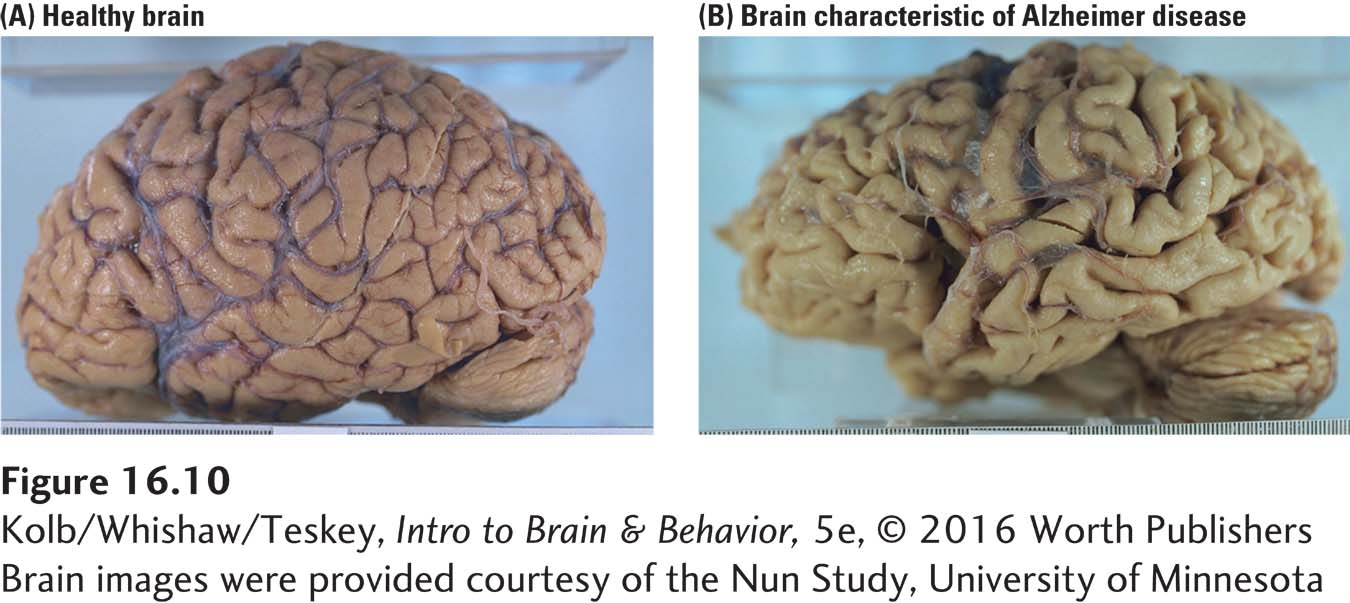
Finally, neocortical changes that correlate with Alzheimer disease are not uniform. As Figure 16-10 shows plainly, the cortex atrophies and can lose as much as one-
589
Areas of most extensive change are the neocortical association areas and the allocortex. The entorhinal cortex is affected earliest and most severely. Entorhinal cortex is the major information relay from the neocortex traveling to the hippocampus and related structures, then back to the neocortex. Entorhinal damage is associated with memory loss, an early and enduring symptom of Alzheimer disease, and is most likely caused by degenerative changes that take place in this area.
Many studies describe cell loss in the cortices of Alzheimer patients, but this finding is disputed. There seems to be a substantial reduction in large neurons, but these cells may shrink rather than disappear. The more widespread cause of cortical atrophy appears to be a loss of dendritic arborization (Figure 16-11).
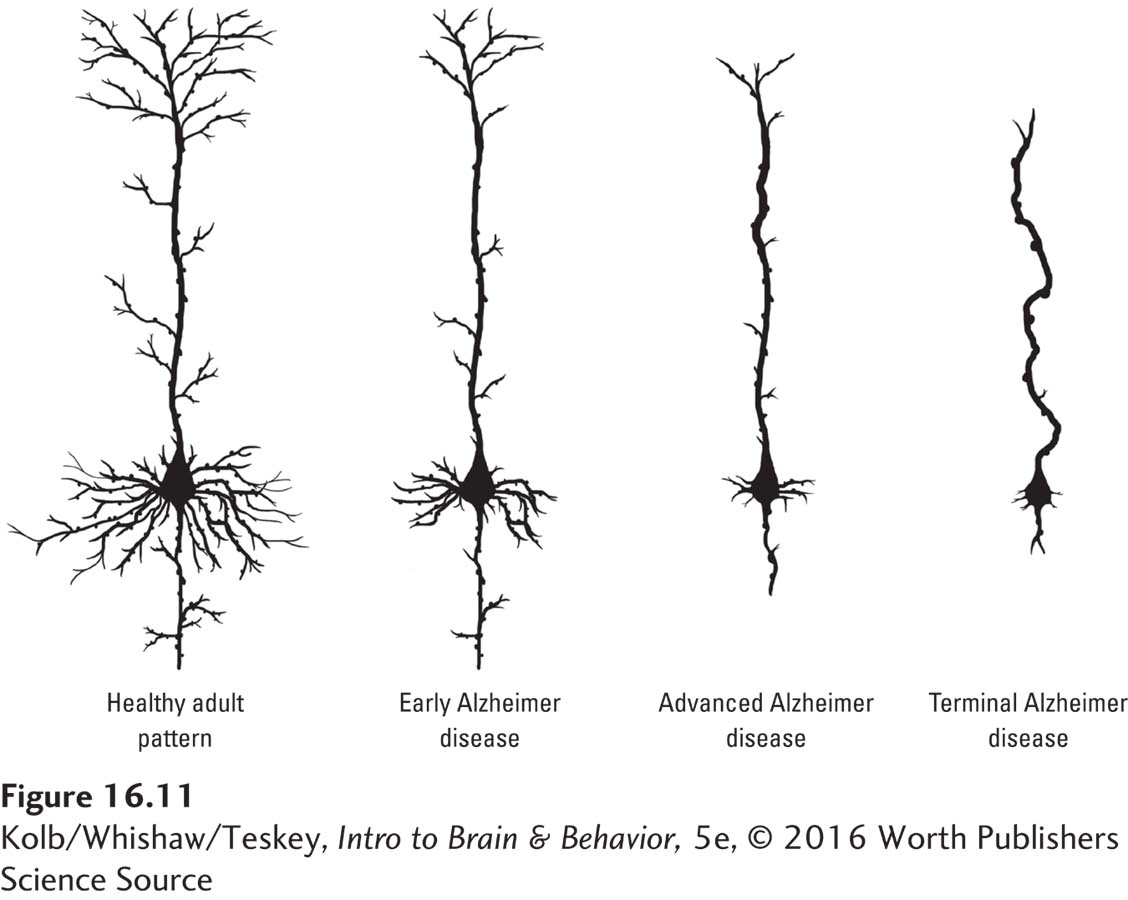
Figure 14-17 shows how glutamate can affect NMDA and AMPA receptors to promote learning by association.
In addition to cell loss and shrinkage, changes take place in the remaining cells’ neurotransmitters. In the 1970s, researchers believed that a treatment paralleling l-dopa treatment of Parkinson disease could be found for Alzheimer disease. The prime candidate neurotransmitter was acetylcholine, and one treatment developed for Alzheimer disease is medication that increases acetylcholine levels in the forebrain. An example, available both orally and as a skin patch, is Exelon, the brand name for rivastigmine, a cholinergic agonist that appears to provide temporary relief from disease progression. Unfortunately, Alzheimer has proved far more complex, because transmitters other than ACh clearly are changed as well. Noradrenaline, dopamine, and serotonin are reduced, as are the NMDA and AMPA receptors for glutamate.
Are All Degenerative Dementias Aspects of a Single Disease?
Neither Parkinson nor Alzheimer disease is related to a single brain structure or region, although dopamine in the case of the former and acetylcholine in the case of the latter seem more affected. Other similarities in their pathology suggest some common neurodegenerative processes. Donald Calne (Calne & Mizuno, 2004) noted that when scientists traveled to Guam at the end of World War II to investigate a reportedly widespread dementia described as similar to Alzheimer disease, they did indeed report a high incidence of Alzheimer. Many years later, Calne and his colleagues, also experts in Parkinson disease, examined the same general group of people and found that they had Parkinson disease. Calne noted that, if you look for Alzheimer symptoms in these people, you find them and miss the Parkinson symptoms. And vice versa.
590
The best-
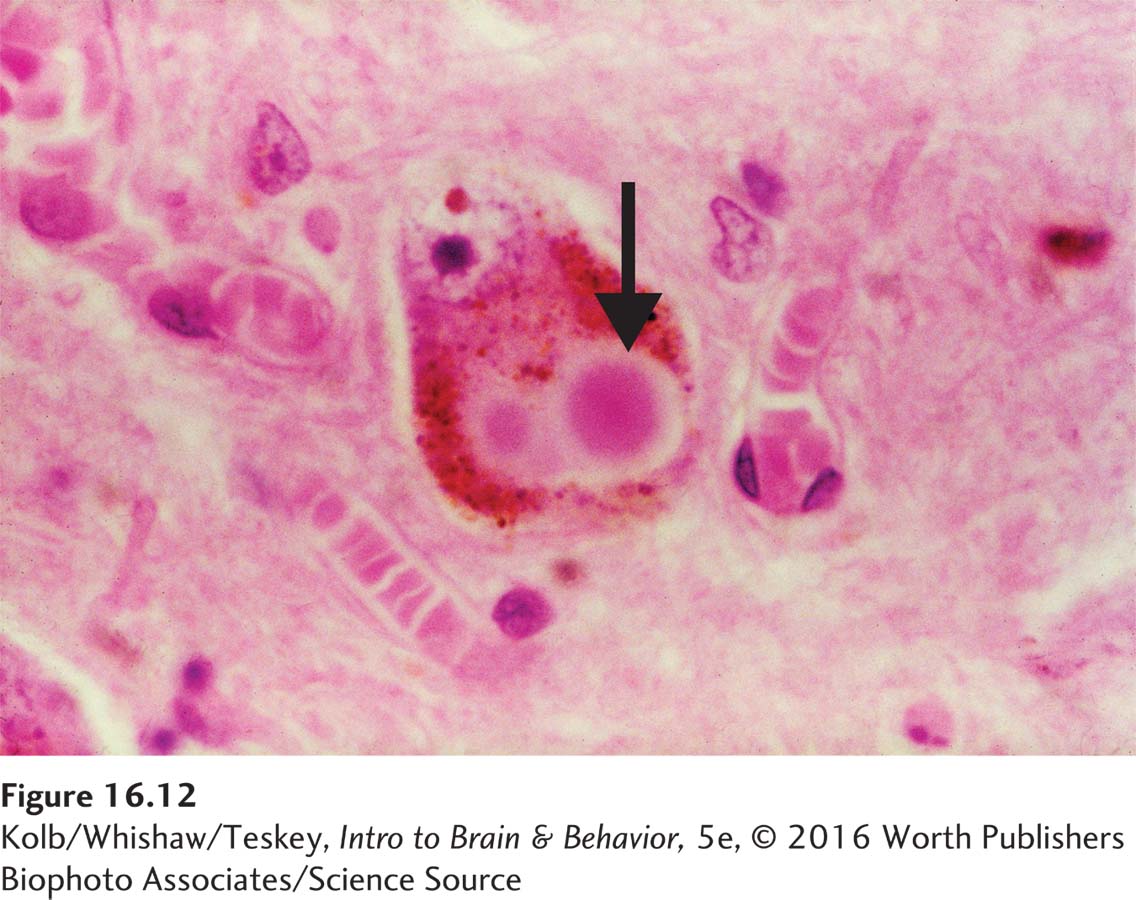
Infection as referred to in the definition is not one caused by casual contact.
Alzheimer and Parkinson symptoms may be similar because both diseases have similar origins. Indeed, the idea that several diseases marked by brain degeneration—
Prions were identified during investigation of various degenerative brain diseases in humans and other animals. Creutzfeldt-
The infectious nature of such conditions was observed in the Fore tribe of Papua New Guinea in the 1950s. A large number of Fore were dying of a muscle-
The infectious agent in these conditions is a prion. Prion proteins are found in healthy cell membranes and may play a role in attaching one cell to another. Prion proteins also bind to metallic ions, for example, copper. The proteins typically fold in a normal configuration but can also misfold (Figure 16-13). The altered configuration causes disease.
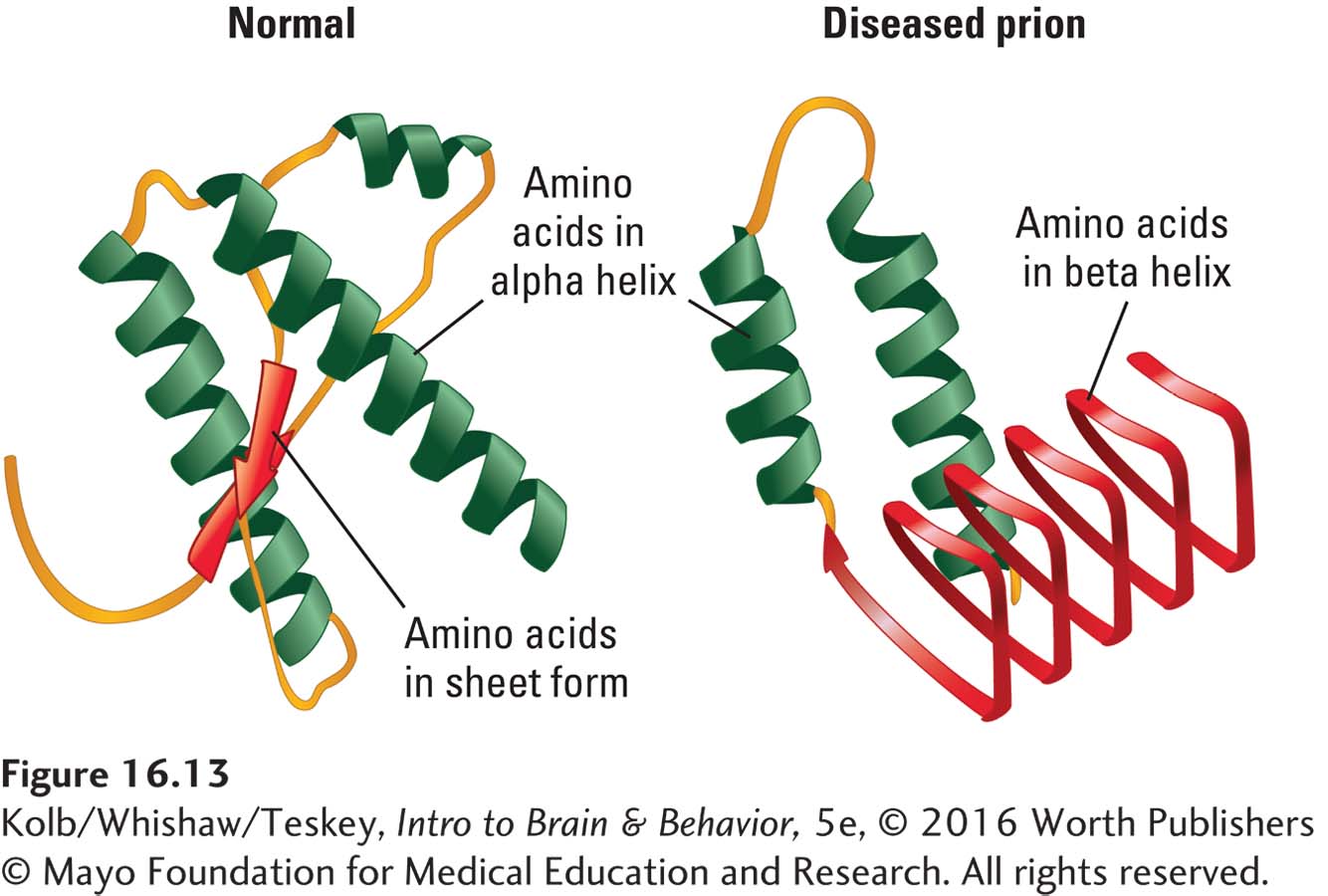
A misfolded prion protein will attach to a healthy prion protein and cause it to misfold. Misfolded prions tend to clump together, forming protein aggregates that eventually result in cell death. Misfolded prions can also infect neighboring brain and body cells, resulting in general brain degeneration and muscle wasting. An infectious prion can pass from one individual to another, and even from one species to another, but only if the normal prion proteins in the two individuals are similar. Investigations show that among several alleles of the gene that produces normal prion proteins, some are more susceptible to misfolding than are others. Individuals with alleles that are not susceptible to misfolding, as are those Fore tribespeople who did not contract kuru, are resistant to prion disease.
591
In Parkinson disease, the misfolded protein is proposed to be alpha synuclein. Its accumulation, largely in substantia nigra cells, is a Lewy body (Olanow & Brundin, 2013). In Alzheimer disease, the accumulating protein forms amyloid plaques and mainly affects cortical cells (Kumar et al., 2015). Misfolded proteins in oligodendroglia may be responsible for the cell demyelination characteristic of MS and ALS (Shi et al., 2015).
The prion theory opens new avenues for investigating degenerative disease treatments, including drugs that remove prions, block prion misfolding, or alter prion forms prone to misfolding (Asante et al., 2015). Selective cattle breeding for prion alleles that do not produce misfolding prevents BSE. Inserting a misfolding-
Age-
Most people who grow old do not become demented, but virtually everyone acquires age-

Noninvasive imaging studies reveal that aging is correlated with decreased white matter volume, probably related to myelin loss. This condition is reparable. There is little evidence of neuronal loss in typical aging, although a reduction in neurogenesis in the hippocampus does occur. Compared with younger people, older people tend to activate larger regions of their attentional and executive networks (parietal and prefrontal cortex) when they perform complex cognitive and executive tasks. This increased activation correlates with reduced performance on tests of working memory as well as tests of attentional and executive tasks.
Two lines of evidence suggest that that age-
View the progression of these microbleeds, or silent strokes, at https://www.youtube.com/watch?v=J3fb0CaDpEk.
William Milberg and his colleagues (e.g., Kuo et al., 2005; Leritz et al., 2011) have explored the idea that aging changes in the brain take place in the context of the entire body’s aging. For example, dementia may reflect a chronic cerebrovascular disorder, marginal high blood pressure. Marginal elevations in blood pressure can lead to cerebral microbleeds, especially in white matter. The cumulative effect of years or even decades of tiny bleeds would lead eventually to increasingly disturbed cognition. The condition may first appear as a mild cognitive impairment (MCI) that slowly progresses, with cumulative microbleeds, toward dementia.
16-3 REVIEW
592
Understanding and Treating Neurological Disorders
Before you continue, check your understanding.
Question 1
As the developed world’s population ages, ____________ disease will become more common than ____________ disease.
Question 2
Even imaging techniques may miss pathology produced by ____________.
Question 3
Interruption of blood to the brain is called ____________ and if prolonged can result in ____________.
Question 4
Although superficially appearing to be very different diseases, Parkinson and Alzheimer have similarities such as ____________ and ____________ that suggest that they may be part of a common disease spectrum.
Question 5
Name two strategies that can reduce or reverse neurological and cognitive decline with aging.
Answers appear in the Self Test section of the book.