4-3 How Neurons Integrate Information
A neuron’s extensive dendritic tree is covered with spines, and through them it can establish more than 50,000 connections from other neurons. Lying between its dendritic tree and axon, a neuron’s cell body, too, can receive multiple connections. Nerve impulses traveling from other neurons to each of these synaptic locations bombard the receiving neuron with excitatory and inhibitory inputs.
Neurons that receive more than one kind of input sum up the information they get.
In the 1960s, John C. Eccles (1965) and his students performed experiments that helped to answer the question, how does the neuron integrate such an enormous array of inputs into a nerve impulse? Rather than recording from the giant axon of a squid, Eccles recorded from the cell bodies of large motor neurons in the vertebrate spinal cord. In doing so, he refined the electrical stimulating and recording techniques first developed for studying squid axons (see Section 4-1). Eccles received the Nobel Prize in Physiology or Medicine in 1963.
Motor neurons, for example, receive input from multiple sources. A spinal cord motor neuron has an extensive dendritic tree with as many as 20 main branches that subdivide numerous times and are covered with dendritic spines. Input from the skin, joints, muscles, spinal cord, and brain make motor cells ideal for studying how a neuron responds to diverse inputs. Each motor neuron sends its axon directly to a muscle. The motor neuron is the final common pathway the nervous system takes to produce behavior.
Excitatory and Inhibitory Postsynaptic Potentials
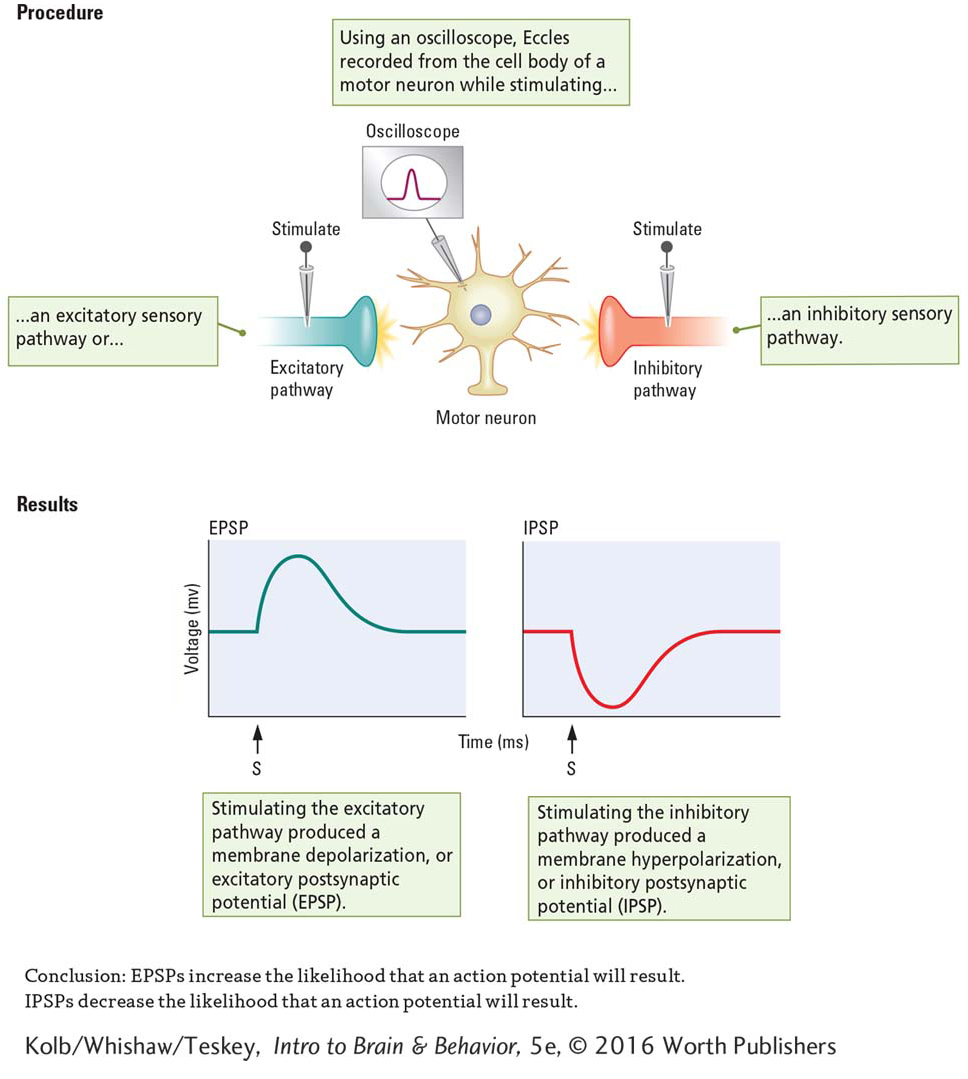
To study motor neuron activity, Eccles inserted a microelectrode into a vertebrate’s spinal cord until the tip was in or right beside a motor neuron’s cell body. He then placed stimulating electrodes on sensory nerve fiber axons entering the spinal cord. By teasing apart the incoming sensory fibers, he was able to stimulate one nerve fiber at a time.
Figure 2-29A diagrams the human spinal cord in cross section.
Experiment 4-1 diagrams the experimental setup Eccles used. As shown at the left in the Procedures section, stimulating some incoming sensory fibers produced a depolarizing graded potential (reduced the charge) on the membrane of the motor neuron to which these fibers were connected. Eccles called these graded potentials excitatory postsynaptic potentials (EPSPs). As graphed at left in the Results section, EPSPs reduce the charge on the membrane toward the threshold level and increase the likelihood that an action potential will result.
At the right in the Procedures section, when Eccles stimulated other incoming sensory fibers, he produced a hyperpolarizing graded potential (increased the charge) on the receiving motor neuron membrane. Eccles called these graded potentials inhibitory postsynaptic potentials (IPSPs). As graphed at the right in the Results section, IPSPs increase the charge on the membrane away from the threshold level and decrease the likelihood that an action potential will result.
128
EXPERIMENT 4-1
Question: How does stimulating a neuron influence its excitability?
Both EPSPs and IPSPs last only a few milliseconds before they decay and the neuron’s resting potential is restored. EPSPs are associated with the opening of sodium channels, which allows an influx of sodium ions. IPSPs are associated with the opening of potassium channels, which allows an efflux of potassium ions (or with the opening of chloride channels, which allows an influx of chloride ions).
A brief video at www.youtube.com/
Although the size of a graded potential is proportional to the intensity of the stimulation, an action potential is not produced on the motor neuron’s cell body membrane even when an EPSP is strongly excitatory. The reason is simple: the cell body membrane of most neurons does not contain voltage-
Summation of Inputs
A motor neuron’s myriad dendritic spines can each contribute to membrane voltage, via either an EPSP or an IPSP. How do these incoming graded potentials interact at its membrane? What happens if two EPSPs occur in succession? Does it matter if the time between them increases or decreases? What happens when an EPSP and an IPSP arrive together?
Temporal Summation
129
If one excitatory pulse is followed some time later by a second excitatory pulse, one EPSP is recorded and after a delay, a second identical EPSP is recorded, as shown at the top left in Figure 4-22.
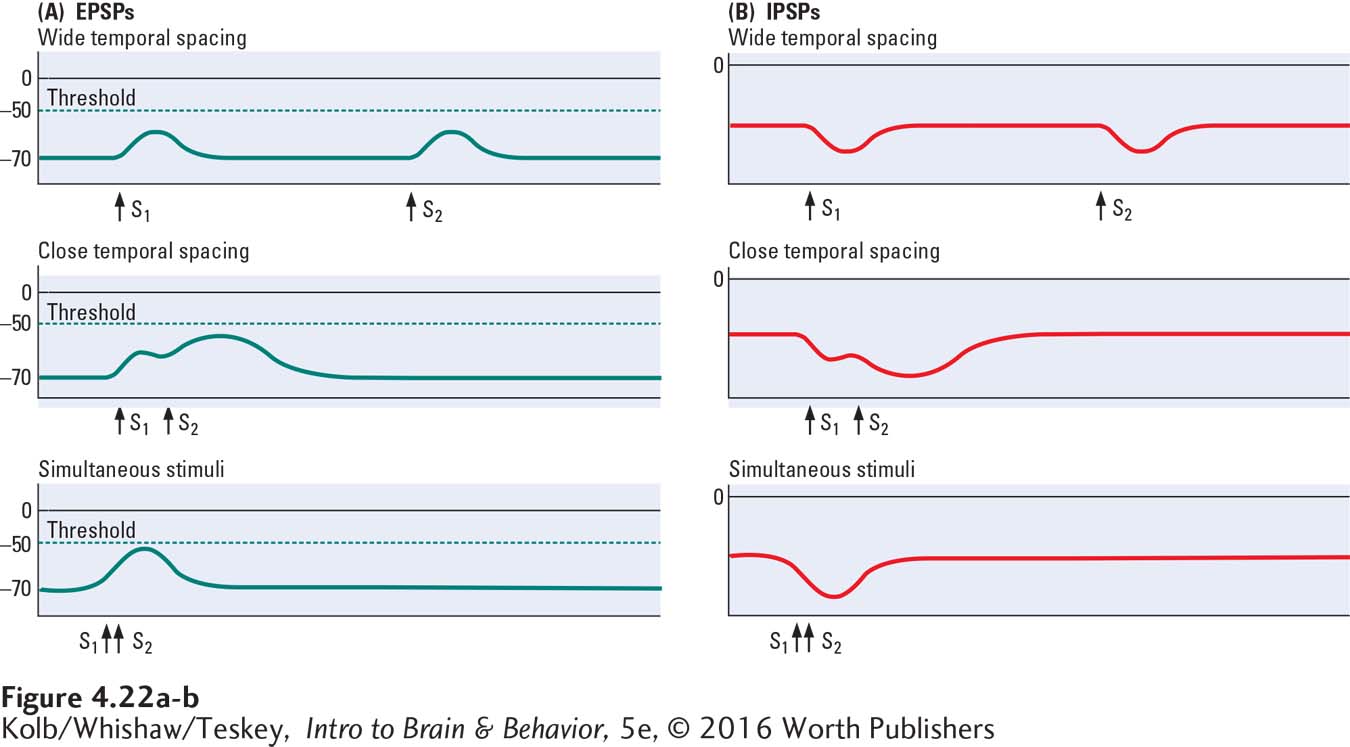
Here, the two excitatory pulses are summed—
Spatial Summation
How does spacing affect inputs to the cell body membrane? By using two recording electrodes (R1 and R2) we can see the effects of spatial relations on the summation of inputs.
If two EPSPs are recorded at the same time but on widely separated parts of the membrane (Figure 4-23A), they do not influence one another. If two EPSPs occurring close together in time are also close together on the membrane, however, they sum to form a larger EPSP (Figure 4-23B). This spatial summation occurs when two separate inputs are very close to one another both on the cell membrane and in time. Similarly, two IPSPs produced at the same time sum if they occur at approximately the same place and time on the cell body membrane but not if they are widely separated.
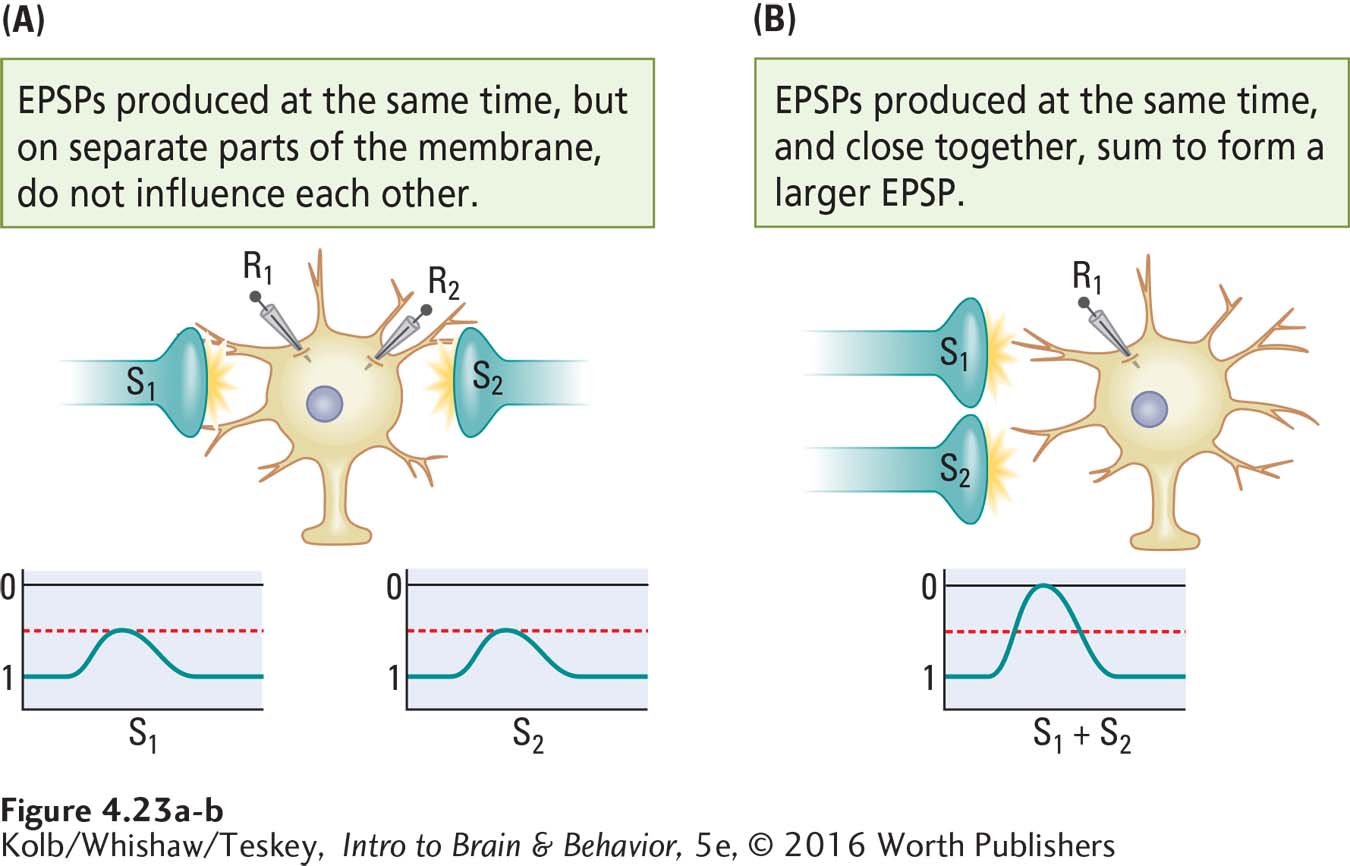
Role of Ions in Summation
Summation is a property of both EPSPs and IPSPs in any combination. These interactions make sense when you consider that ion influx and efflux are being summed. The influx of sodium ions accompanying one EPSP is added to the influx of sodium ions accompanying a second EPSP if the two occur close together in time and space. If the two influxes are remote in time or in space or in both, no summation is possible.
130
The same is true regarding effluxes of potassium ions. When they occur close together in time and space, they sum; when they are far apart in either or both ways, there is no summation. The patterns are identical for an EPSP and an IPSP. The influx of sodium ions associated with the EPSP is added to the efflux of potassium ions associated with the IPSP, and the difference between them is recorded as long as they are spatially and temporally close together. If, on the other hand, they are widely separated in time or in space or in both, they do not interact and there is no summation.
A neuron with thousands of inputs responds no differently from one with only a few inputs. It democratically sums all inputs that are close together in time and space. The cell body membrane, therefore, always indicates the summed influences of multiple temporal and spatial inputs. And so a neuron can be said to analyze its inputs before deciding what to do. The ultimate decision is made at the initial segment, the region on the axon that initiates the action potential.
Voltage-
Unlike the cell body membrane, the axon is rich in voltage-
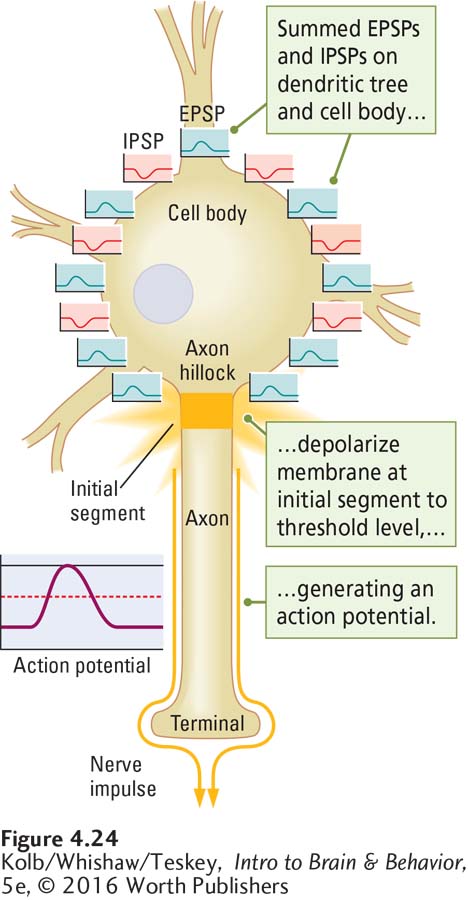
To produce an action potential, the summed graded potentials—
Many neurons have extensive dendritic trees, but dendrites and dendritic branches do not have many voltage-
The Versatile Neuron
The hippocampus (from the Greek meaning seahorse because its shape is similar) participates in aspects of memory and is vulnerable to stress (see Section 6-5).
Dendrites collect information as graded potentials (EPSPs and IPSPs), and the initial segment initiates discrete action potentials delivered to other target cells via the axon. Exceptions to this picture of how a neuron works do exist. For example, some cells in the developing hippocampus can produce additional action potentials, called giant depolarizing potentials, when the cell would ordinarily be refractory. It is thought that giant depolarizing potentials aid in developing the brain’s neural circuitry (Mohajerani & Cherubini, 2006).
We explore the neuronal basis of learning in Sections 5-4 and 14-4.
Because the cell body membrane does not contain voltage-
131
Section 7-1 describes the promise of optogenetics for neuroscience research and for clinical applications.
The neurons of some nonmammalian species have no dendritic branches. And some ion channels, rather than responding to voltage, respond to light by opening and allowing ions to pass. The many differences among neurons suggest that the nervous system capitalizes on structural and functional modifications to produce adaptive behavior in each species. In research to determine the neuron’s specific functions, neuroscientists have incorporated into certain types of neurons ion channels that respond to light, as described in Research Focus 4-3, Optogenetics and Light-Sensitive Channels.
RESEARCH FOCUS 4-3
Optogenetics and Light-
Membrane channels that are responsive to light have been discovered in nonmammalian animal species. Using the transgenic technique of optogenetics, researchers have successfully introduced light-
Optogenetics combines genetics and light to control targeted cells in living tissue. Here we examine how introducing different light-
One class of light-
Halorhodopsin (NpHR) is a light-
The movements of worms, fruit flies, and mice with genetically introduced light-
C. elegans is popular in neuroscience experiments because it is transparent and has a simple nervous system. It is also the first species to have all its neurons, synapses, and genome completely described. The illustration shows (A) the normal movements of C. elegans and the light-
Using optogenetic techniques, light-
Also encouraging are the results of a study suggesting that impaired vision due to the loss of the light-
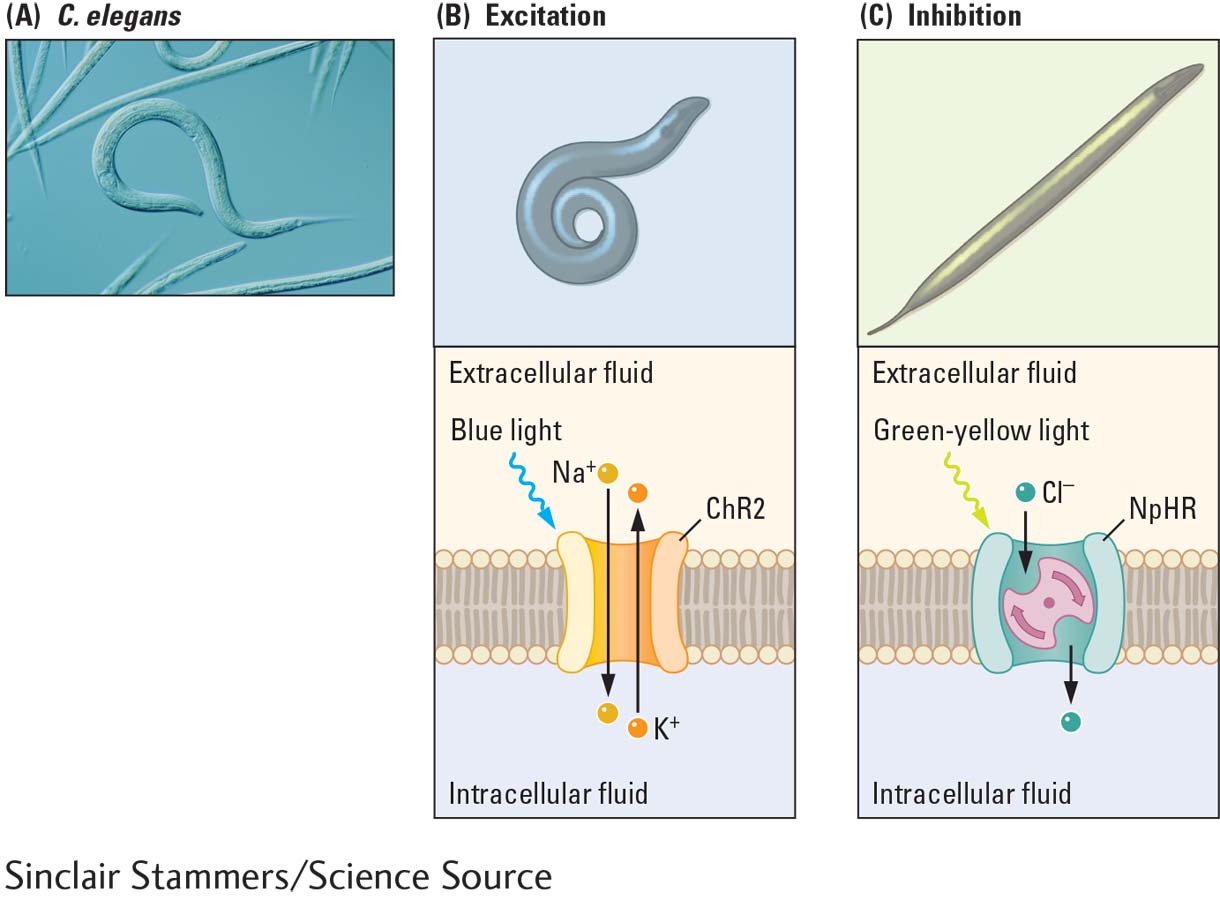
(A) Normal movements of C. elegans. (B) When light-
4-3 REVIEW
132
How Neurons Integrate Information
Before you continue, check your understanding.
Question 1
Graded potentials that decrease the charge on the cell membrane, moving it toward the threshold level, are called ________ because they increase the likelihood that an action potential will occur. Graded potentials that increase the charge on the cell membrane, moving it away from the threshold level, are called ______ because they decrease the likelihood that an action potential will result.
Question 2
EPSPs and IPSPs that occur close together in both _______ and ______ are summed. This is how a neuron ______ the information it receives from other neurons.
Question 3
The membrane of the ________ does not contain voltage-
Question 4
Explain what happens during back propagation.
Answers appear in the Self Test section of the book.