6-1 Principles of Psychopharmacology
173
Drugs are chemical compounds administered to bring about some desired change in the body and brain. Drugs are usually used to diagnose, treat, or prevent illness; to relieve pain and suffering; or to improve some adverse physiological condition. In this chapter, we focus on psychoactive drugs—substances that alter mood, thought, or behavior; are used to manage neuropsychological illness; and may be abused. We also consider psychoactive drugs that can act as toxins, producing sickness, brain damage, or death.
Drug Routes into the Nervous System
To be effective, a psychoactive drug has to reach its target in the nervous system. The way a drug enters and passes through the body to reach its target is called its route of administration. Drugs can be administered orally, inhaled into the lungs, administered rectally in a suppository, absorbed from patches applied to the skin or mucous membranes, or injected into the bloodstream, into a muscle, or even into the brain. Figure 6-1 illustrates some of these routes of drug administration and summarizes the characteristics of drugs that allow them to pass through various barriers to reach their targets.
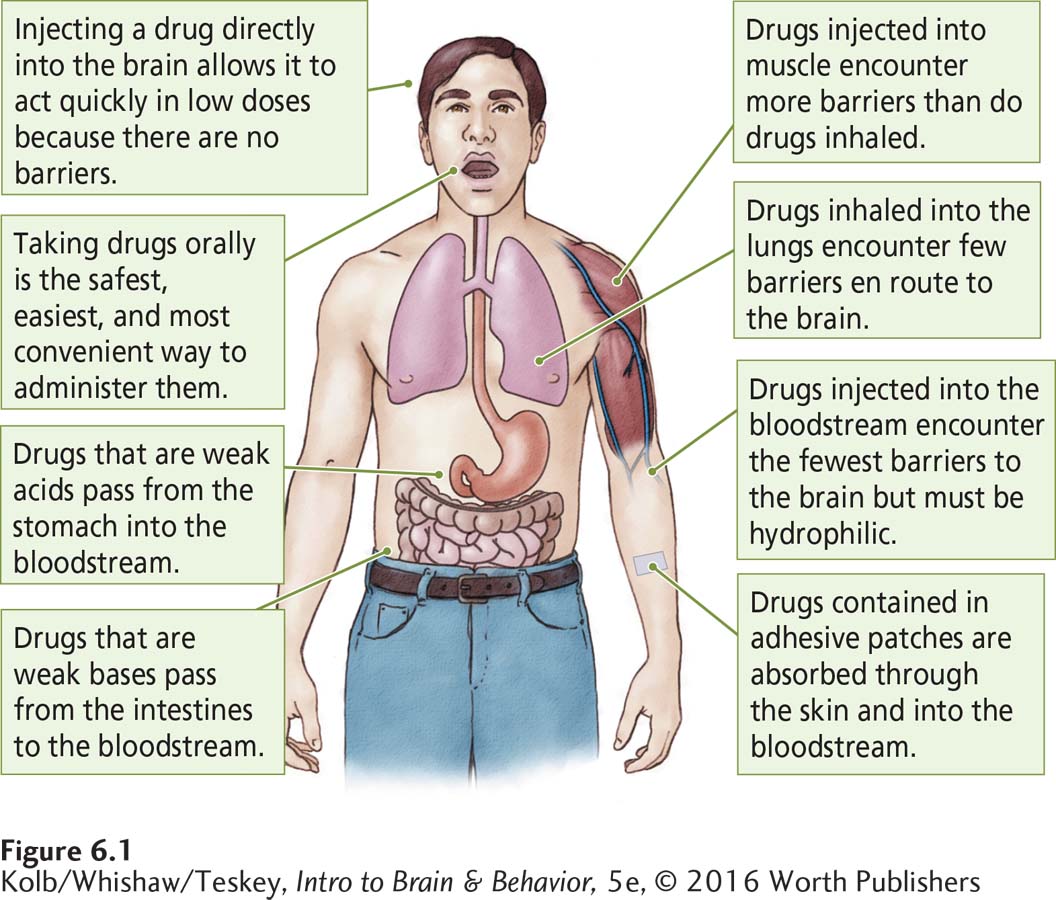
Oral administration is easy and convenient but is nonetheless a complex route. To reach the bloodstream, an ingested drug must first be absorbed through the lining of the stomach or small intestine. Drugs in liquid form are absorbed more readily. Drugs taken in solid form are not absorbed unless the stomach’s gastric juices can dissolve them. Some drugs may be destroyed or altered by enzymes in the gastrointestinal tract’s microbiome. Whether a drug is an acid or a base influences its absorption.
Once absorbed by the stomach or intestine, the drug must enter the bloodstream. This leg of the journey requires that the drug have additional properties. Because blood has a high water concentration, the drug must be water-
Drugs administered as gases or aerosols penetrate the cell linings of the respiratory tract easily and are absorbed across these membranes into the bloodstream nearly as quickly as they are inhaled. Thus, they reach the bloodstream by circumventing the barriers in the digestive system. When administered as a gas or in smoke, drugs of abuse, including nicotine, cocaine, and marijuana, are similarly absorbed.
Our largest organ, the skin, has three cell layers designed to be a protective body coat. Some small-
1000 μg = 1 mg (milligram)
With each obstacle eliminated en route to the brain, a drug’s dosage can be reduced by a factor of 10. For example, 1000 micrograms (μg; 1 μg is equal to one-
174
This math is well known to sellers and users of illicit drugs. Drugs prepared for inhalation or intravenous injection are much cheaper per dose, because the amount required is so much smaller than that needed for an effective oral dose.
Revisiting the Blood–
Figures 4-7 and 4-8 illustrate ion diffusion and concentration and voltage gradients.
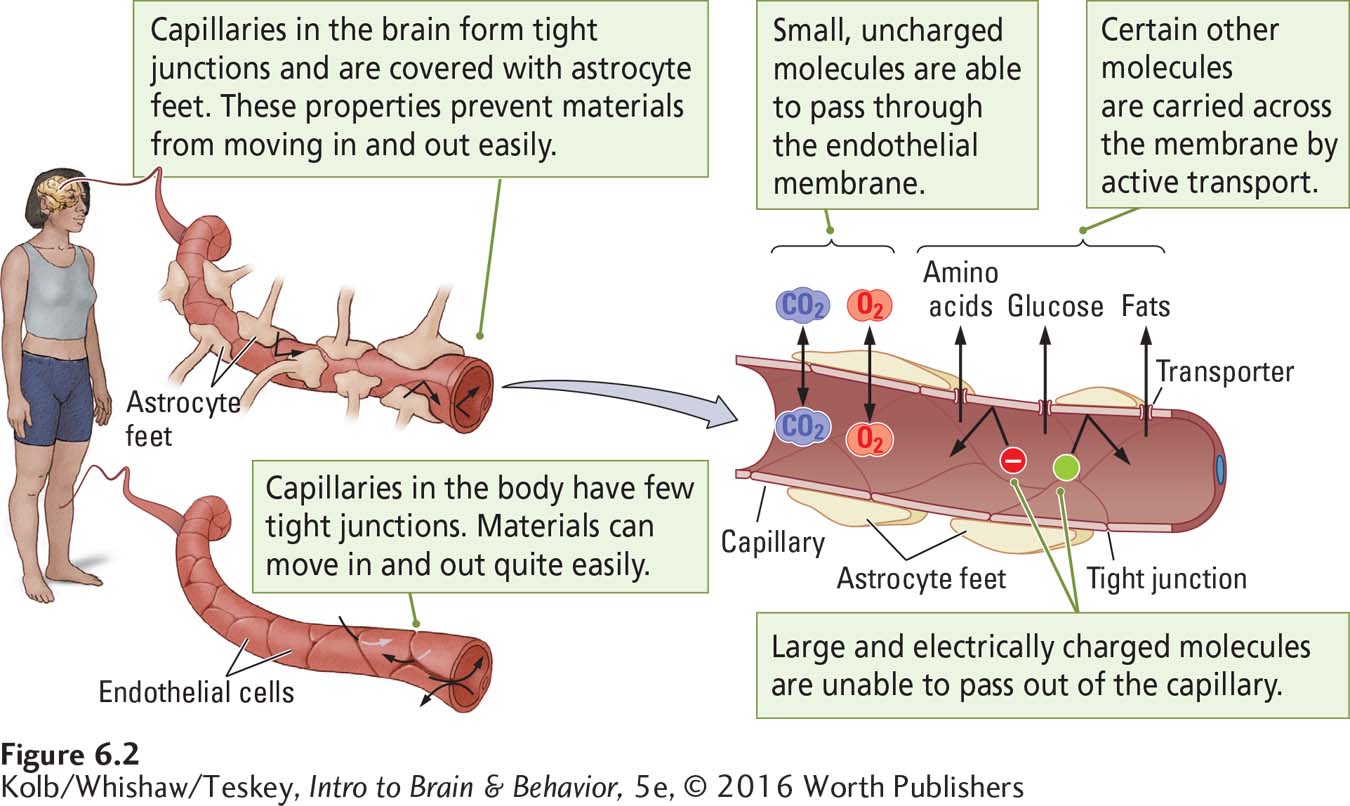
The body presents barriers to the internal movement of drugs: cell membranes, capillary walls, and the placenta. The passage of drugs across capillaries in the brain is made difficult by the blood–
The brain has a rich capillary network. None of its neurons is farther than about 50 micrometers (μm; 1 μm is equal to one-
Figure 6-2 also shows that brain capillaries’ endothelial cells are surrounded by the end feet of astrocytes attached to the capillary wall, covering about 80 percent of it. The glial cells provide a route for the exchange of food and waste between capillaries and the brain’s extracellular fluid and from there to other cells, shown at the right in Figure 6-2.
Section 13-2 details the pineal gland’s pacemaking function.
The cells of capillary walls in the three brain regions shown in Figure 6-3 lack a blood–
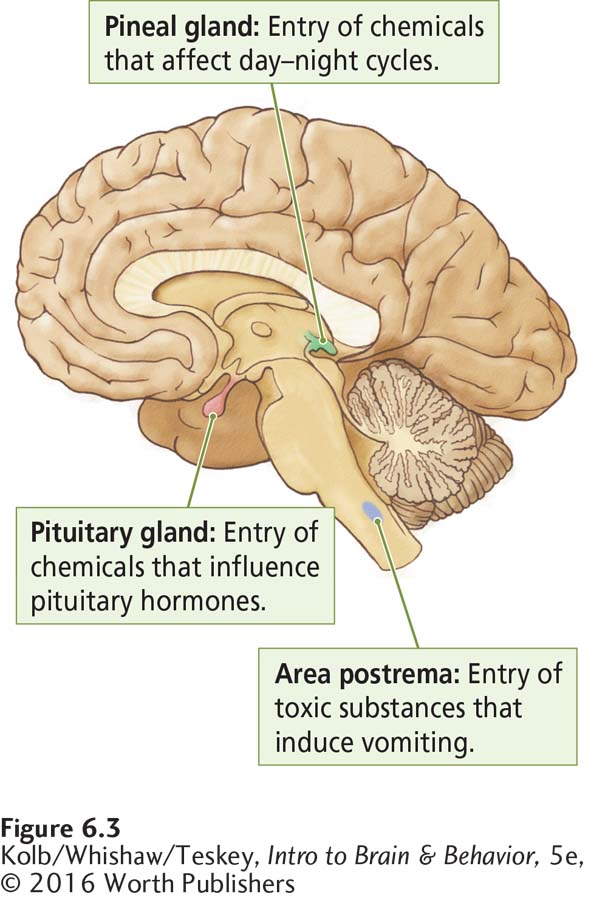
175
To carry out its work, the brain needs, among other substances, oxygen and glucose for fuel and amino acids to build proteins. Fuel molecules reach brain cells from the blood, just as carbon dioxide and other waste products are excreted from brain cells into the blood. Molecules of these vital substances cross the blood–
Small molecules such as oxygen and carbon dioxide can pass through the endothelial membrane.
Complex molecules of glucose, amino acids, and other food components are carried across the membrane by active transport systems or ion pumps—
transporter proteins specialized to convey a particular substance.
Few psychoactive drug molecules are sufficiently small or have the correct chemical structure to gain access to the CNS. An important property possessed by those few drugs that have CNS effects, then, is an ability to cross the blood–
How the Body Eliminates Drugs
After a drug is administered, the body soon begins to break it down (catabolize) and remove it. Drugs are diluted throughout the body and are sequestered in many regions, including fat cells. They are also catabolized throughout the body, including in the kidneys and liver, and in the intestine by bile. They are excreted in urine, feces, sweat, breast milk, and exhaled air. Drugs developed for therapeutic purposes are usually designed not only to increase their chances of reaching their targets but also to enhance their survival time in the body.
Catabolic processes break down; metabolic processes build up.
The liver is especially active in catabolizing drugs. Owing to a family of enzymes involved in drug catabolism, the cytochrome P450 enzyme family (some are also present in the gastrointestinal tract microbiome), the liver is capable of breaking down many different drugs into forms more easily excreted from the body. Substances that cannot be catabolized or excreted can build up in the body and become toxic. The metal mercury, for instance, is not easily eliminated and can produce severe neurological effects.
Drugs eliminated from the body and discharged into the environment are extensive and problematic. They may be reingested, via food and water, by many animal species, including humans (Brown et al., 2015). Some may affect fertility, embryonic development, even the physiology and behavior of adult organisms. The solution is redesigning waste management systems to remove by-
Drug Action at Synapses: Agonists and Antagonists
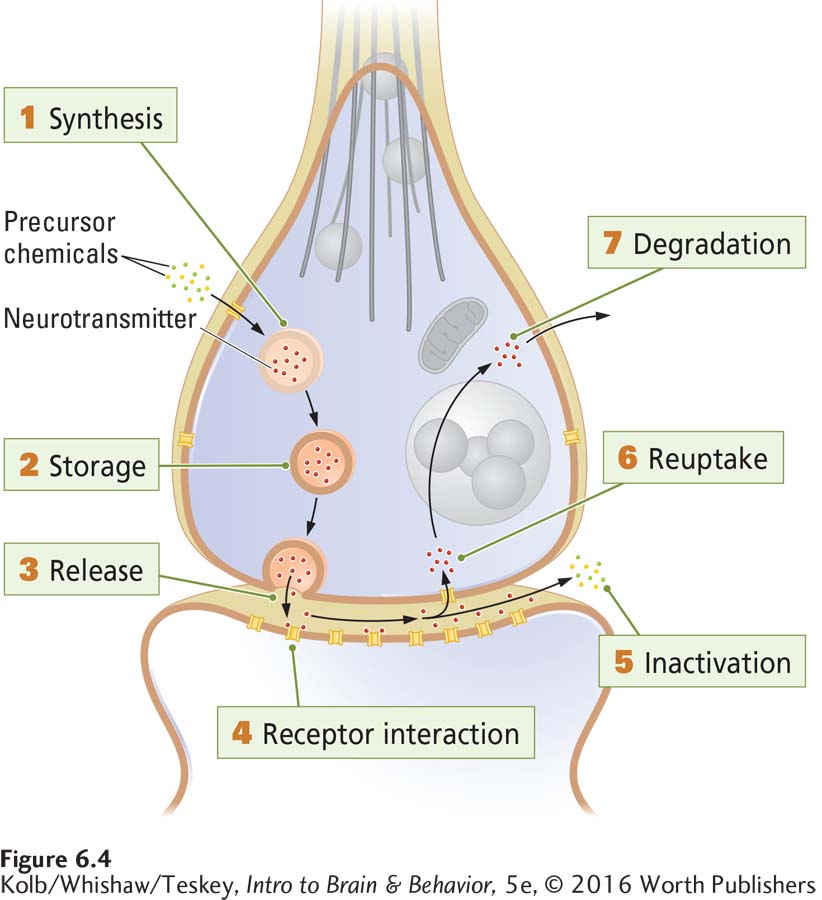
Most drugs that produce psychoactive effects work by influencing chemical reactions at synapses. So to understand how drugs work, we must explore the ways they modify synaptic actions. Figure 6-4 summarizes seven major steps in neurotransmission at a synapse—
Synthesis of the neurotransmitter can take place in the cell body, the axon, or the terminal.
Storage of the neurotransmitter is in granules or in vesicles or in both.
Release of the transmitter is from the terminal’s presynaptic membrane into the synapse.
Receptor interaction takes place in the postsynaptic membrane, as the transmitter acts on an embedded receptor.
Inactivation of excess neurotransmitter occurs at the synapse.
Reuptake into the presynaptic terminal for reuse is one outcome.
Degradation of excess neurotransmitter by synaptic mechanisms and removal of unneeded by-
products from the synapse is the other outcome.
176
Ultimately, a drug that affects any of these synaptic functions either increases or diminishes neurotransmission. Drugs that increase neurotransmission are classified as agonists; drugs that decrease neurotransmission are classified as antagonists. To illustrate, consider a typical synapse: the acetylcholine synapse between motor neurons and muscles.
An Acetylcholine Synapse: Examples of Drug Action
Figure 4-26 details the structure and action of ACh at a neuromuscular synapse.
Figure 6-5 shows how some drugs and toxins act as agonists or antagonists at the ACh synapse on skeletal muscles. ACh agonists excite muscles, increasing muscle tone, whereas ACh antagonists inhibit muscles, decreasing muscle tone. Some of these substances may be new to you, but you have probably heard of others. If you know their effects at the ACh synapse, you can understand the relationships between these substances’ neurochemical actions and their behavioral effects.
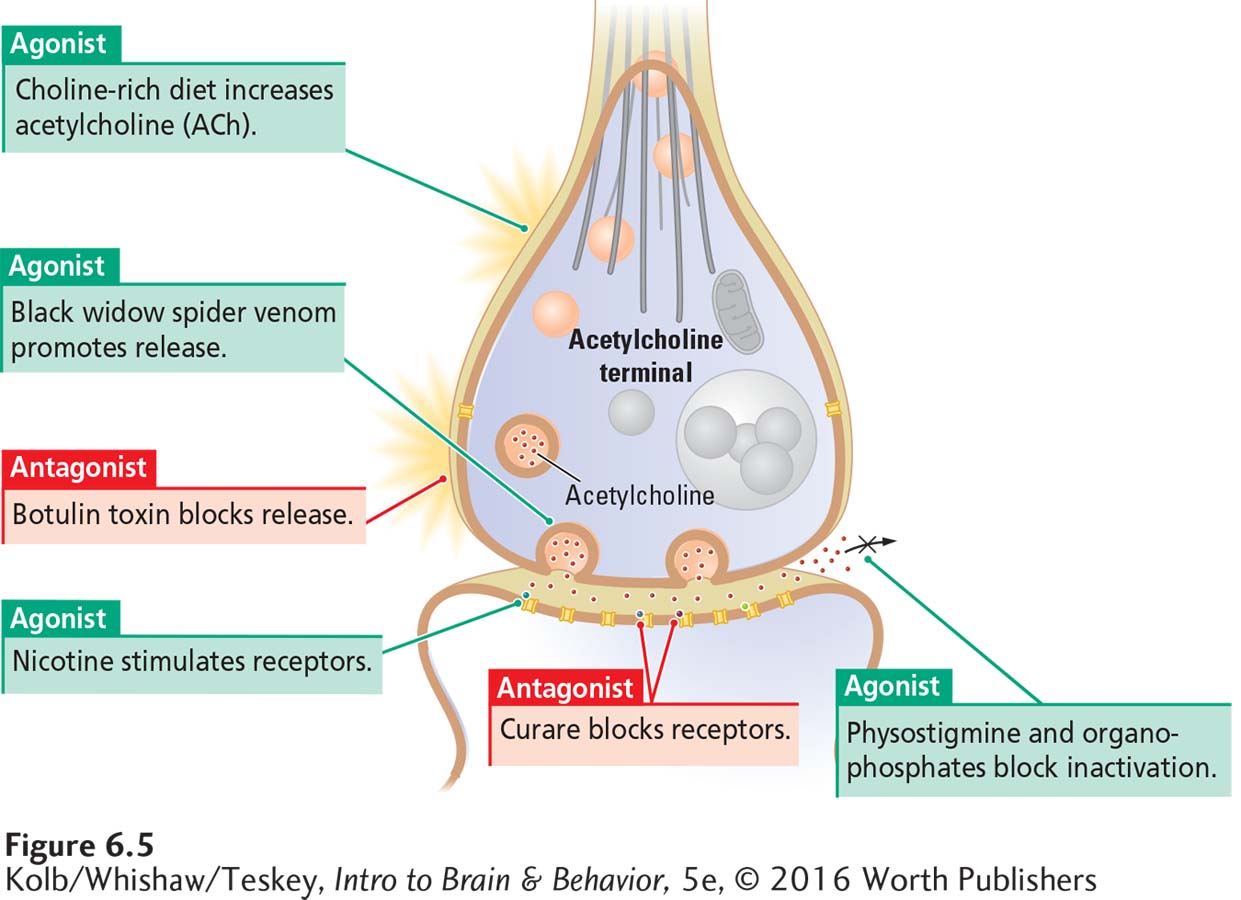
Figure 6-5 includes two toxins that influence ACh release from the axon terminal. Black widow spider venom acts as an agonist by promoting ACh release to excess. A black widow spider bite does not inject enough drug to paralyze a person, though a victim may feel some muscle weakness.
Botulinum toxin, or botulin, is the poisonous agent in improperly processed canned goods. An antagonist, it blocks ACh release, an effect that can last for weeks to months. Severe poisoning can paralyze both movement and breathing and so cause death.
Focus 11-2 describes the causes and range of outcomes for cerebral palsy.
Botulin has medical uses. Injected into a muscle, it can selectively paralyze the muscle. This action makes it useful for blocking excessive and enduring muscular twitches or contractions, including the spasms that make movement difficult, for example in people with cerebral palsy. Under the trade name Botox, botulin is also used cosmetically to paralyze facial muscles that cause wrinkling.
As illustrated in Section 5-3, a single main receptor serves the sympathetic nervous system: the nicotinic ACh receptor (nAChR).
Figure 6-5 also shows two drugs that act on ACh receptors. Nicotine’s molecular structure is similar enough to that of ACh to allow nicotine to fit into ACh receptors’ binding sites, where it acts as an agonist. Curare acts as an ACh antagonist by occupying cholinergic receptors and so preventing ACh from binding to them. Once introduced into the body, curare acts quickly and is cleared from the body in a few minutes. Large doses, however, arrest movement and breathing for a period sufficient to result in death.
177
Early European explorers of South America discovered that the indigenous peoples living along the Amazon River in South America killed small animals using arrowheads coated with curare prepared from the seeds of a plant. The hunters did not poison themselves when eating the animals, because ingested curare cannot pass from the gut into the body. Many curarelike drugs have been synthesized. Some are used to briefly paralyze large animals for examination or tagging for identification. You have probably seen this use of these drugs in wildlife videos. Skeletal muscles are more sensitive to curarelike drugs than are respiratory muscles; an appropriate dose paralyzes an animal’s movement temporarily but allows it to breathe.
Figure 5-10 illustrates ACh synthesis and how AChE breaks it down.
The final drug action shown in Figure 6-5 is that of physostigmine, an agonist that inhibits acetylcholinesterase (AChE), the enzyme that breaks down ACh, thus increasing the amount available in the synapse. Physostigmine, obtained from an African bean, is used as a poison by hunters.
In myasthenia gravis, muscle receptors lose their sensitivity to motor neuron messages, as illustrated in Section 4-4.
Large doses of physostigmine can be toxic because they produce excessive excitation of the neuromuscular synapse, disrupting movement and breathing. In small doses, however, physostigmine is used to treat myasthenia gravis, a condition of muscular weakness in which muscle receptors are less than normally responsive to ACh. Physostigmine’s action is short lived, lasting only a few minutes or at most a half hour.
The Basics, Section 1-3, charts nervous system evolution in the animal kingdom.
Organophosphate compounds bind irreversibly to AChE and consequently allow a toxic buildup of ACh in the synaptic space. Many insecticides and chemical weapons are organophosphates. Insects use glutamate as a neurotransmitter at the nerve–
Does a drug or toxin that affects neuromuscular synapses also affect ACh synapses in the brain? That depends on whether the substance can cross the blood–
Tolerance
In tolerance, as in habituation, learning takes place when the response to a stimulus weakens with repeated presentations (see Experiment 5-2).
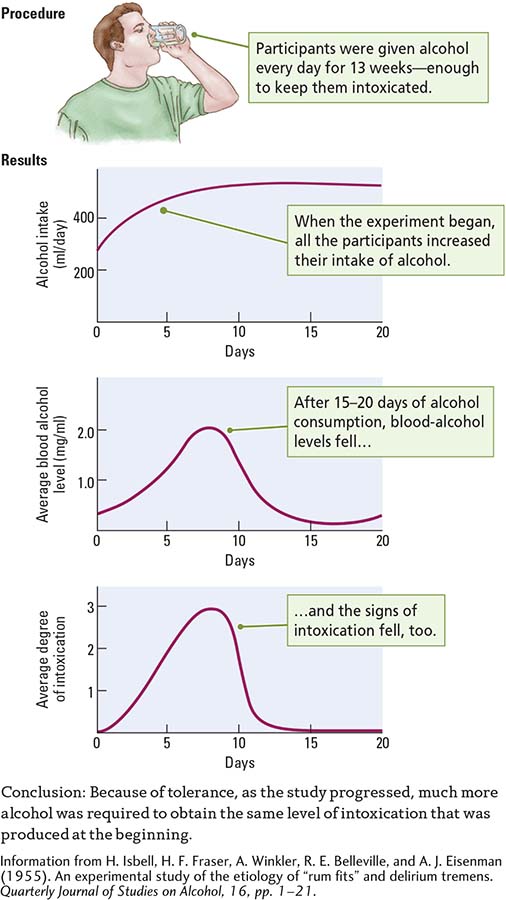
Tolerance is a decreased response to a drug with repeated exposure. Harris Isbell and coworkers (1955) conducted an experiment that, while questionable by today’s ethical standards, did suggest how tolerance comes about. The researchers gave volunteers in a prison enough alcohol daily in a 13-
When the experiment began, the participants showed rapidly rising blood alcohol levels and behavioral signs of intoxication, as shown in the Results section of Experiment 6-1, on page 178. Between the twelfth and twentieth days of alcohol consumption, however, blood alcohol and the signs of intoxication fell, even though the participants maintained their alcohol intake. Thereafter, blood alcohol levels and signs of intoxication fluctuated; one did not always correspond to the other. A relatively high blood alcohol level was sometimes associated with a low outward appearance of intoxication. Why?
The three results were the products of three kinds of tolerance, each much more likely to develop with repeated drug use:
In metabolic tolerance, the number of enzymes needed to break down alcohol in the liver, blood, and brain increases. As a result, any alcohol consumed is metabolized more quickly, so blood alcohol levels fall.
178
In cellular tolerance, brain cell activities adjust to minimize the effects of alcohol in the blood. Cellular tolerance can help explain why the behavioral signs of intoxication may be so low despite a relatively high blood alcohol level.
Learned tolerance explains a drop in outward signs of intoxication. As people learn to cope with the demands of living under the influence of alcohol, they may no longer appear intoxicated.
EXPERIMENT 6-1
Question: Will the consumption of alcohol produce tolerance?
Does it surprise you that learning plays a role in alcohol tolerance? It has been confirmed in many studies, including a description of the effect first reported by John Wenger and his coworkers (1981). They trained rats to prevent electric foot shocks as they walked on a narrow conveyor belt sliding over an electrified grid. One group of rats received alcohol after training in walking the belt; another group received alcohol before training. A third group received training only, and a fourth group received alcohol only.
After several days’ exposure to their respective conditions, all groups received alcohol before a walking test. The rats that had received alcohol before training performed well, whereas those that had received training and alcohol separately performed just as poorly as those that had never had alcohol or those that had not been trained. Despite alcohol intoxication, then, animals can acquire the motor skills needed to balance on a narrow belt. With motor experience, they can learn to compensate for being intoxicated.
Sensitization
Drug tolerance is much more likely to develop with repeated use than with periodic use, but tolerance does not always follow repeated exposure to a drug. Tolerance resembles habituation in that the response to the drug weakens with repeated presentations. The drug user may have the opposite reaction, sensitization—
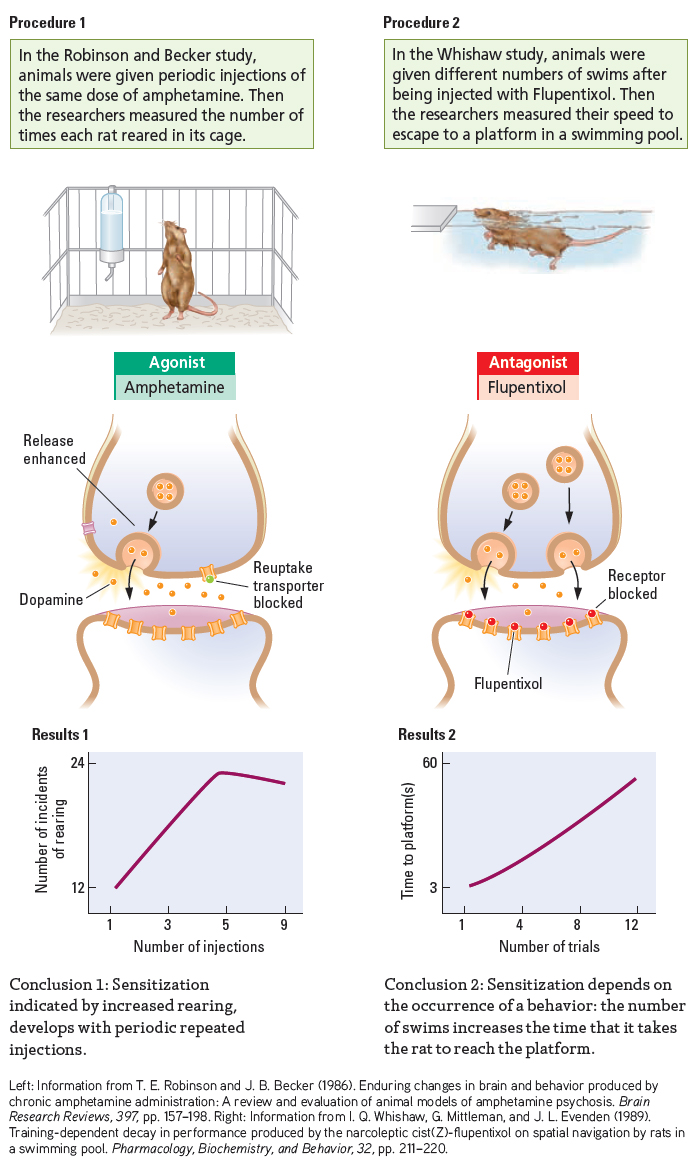
To demonstrate drug sensitization, Terry Robinson and Jill Becker (1986) isolated rats in observation boxes and recorded their reactions to an injection of amphetamine, which stimulates dopamine receptors. Every 3 or 4 days, the investigators injected the rats and found their motor activities—
Experiment 5-3 describes sensitization at the level of neurons and synapses. Section 14-4 relates sensitization to neuroplasticity and learned addictions.
The increased motor activity on successive tests was not due to the animals becoming comfortable with the test situation. Control animals that received no drug failed to display a similar escalation. Administering the drug to rats in their home cages did not affect activity in subsequent tests, either. Moreover, the sensitization to amphetamine was enduring. Even when two injections were separated by months, the animals still showed an escalation of motor behavior. Even a single exposure to amphetamine produced sensitization.
179
EXPERIMENT 6-2
Question: Does the injection of a drug always produce the same behavior?
180
Sensitization is not always characterized by an increase in an elicited behavior but may also manifest as a progressive decrease in behavior. Ian Whishaw and his colleagues (1989) administered flupentixol, a drug that blocks dopamine receptors, to rats that had been well trained in a swimming task. As illustrated in Results 2 of Experiment 6-2, the rats displayed such a marked trial-
The neural basis of sensitization lies in part in changes at the synapse. Studies on the dopamine synapse after sensitization to amphetamine show more dopamine released from the presynaptic terminal in sensitized animals. Sensitization can also be associated with changes in receptor numbers on the postsynaptic membrane, in the rate of transmitter metabolism in the synaptic space, in transmitter reuptake by the presynaptic membrane, and in the number and size of synapses.
Another basis of sensitization is learned. Animals show a change in learned responses to environmental cues as sensitization progresses. Consequently, sensitization is difficult to achieve in an animal tested in its home cage. In the Whishaw group’s experiment, administering flupentixol to rats left in their home environment did not influence their performance in subsequent swim tests.
Sabina Fraioli and her coworkers (1999) gave amphetamine to two groups of rats and recorded the behavioral responses to successive injections. One group of rats lived in the test apparatus, so for that group, home was the test box. The other group was taken out of their home cage and placed in the test box for each day’s experimentation. The home group showed no sensitization to amphetamine, whereas the out group displayed robust sensitization.
At least part of the explanation of the home–
Sensitization is relevant to understanding some psychopharmacological effects of drugs:
Focus 8-5 relates the possible origin of schizophrenia and its progress.
Many drug therapies, including those for the psychiatric disorder schizophrenia, must be taken for several weeks before they produce beneficial effects. Possibly sensitization underlies the development of these beneficial effects.
Sensitization is related to drug dependence. Before a person becomes dependent on or addicted to a drug, he or she must be sensitized by numerous experiences with the drug away from the home environment.
Life experiences, especially stressful ones, can produce effects resembling sensitization that prime the nervous system for addiction (Roberts et al., 2015).
6-1 REVIEW
Principles of Psychopharmacology
Before you continue, check your understanding.
Question 1
_______, substances that produce changes in behavior by acting on the nervous system, are one subject of _______, the study of how drugs affect the nervous system and behavior.
Question 2
Perhaps the most important obstacle on a psychoactive drug’s journey between its entry into the body and its action at a target is the _______, which generally allows only substances needed for nourishment to pass from the capillaries into the _______.
181
Question 3
Most drugs that have psychoactive effects influence chemical reactions at neuronal _______. Drugs that influence communication between neurons do so by acting either as _______ (increasing the effectiveness of neurotransmission) or as _______ (decreasing the effectiveness of neurotransmission).
Question 4
Behavior may change with the repeated use of a psychoactive drug. These changes include _______ and _______, in which the effect of the drug decreases or increases, respectively, with repeated use.
Question 5
The body eliminates drugs through _______, _______, _______, _______, and _______.
Question 6
Describe briefly how tolerance and sensitization might affect someone who uses cognitive enhancers occasionally (a) at home or (b) at work.
Answers appear in the Self Test section of the book.