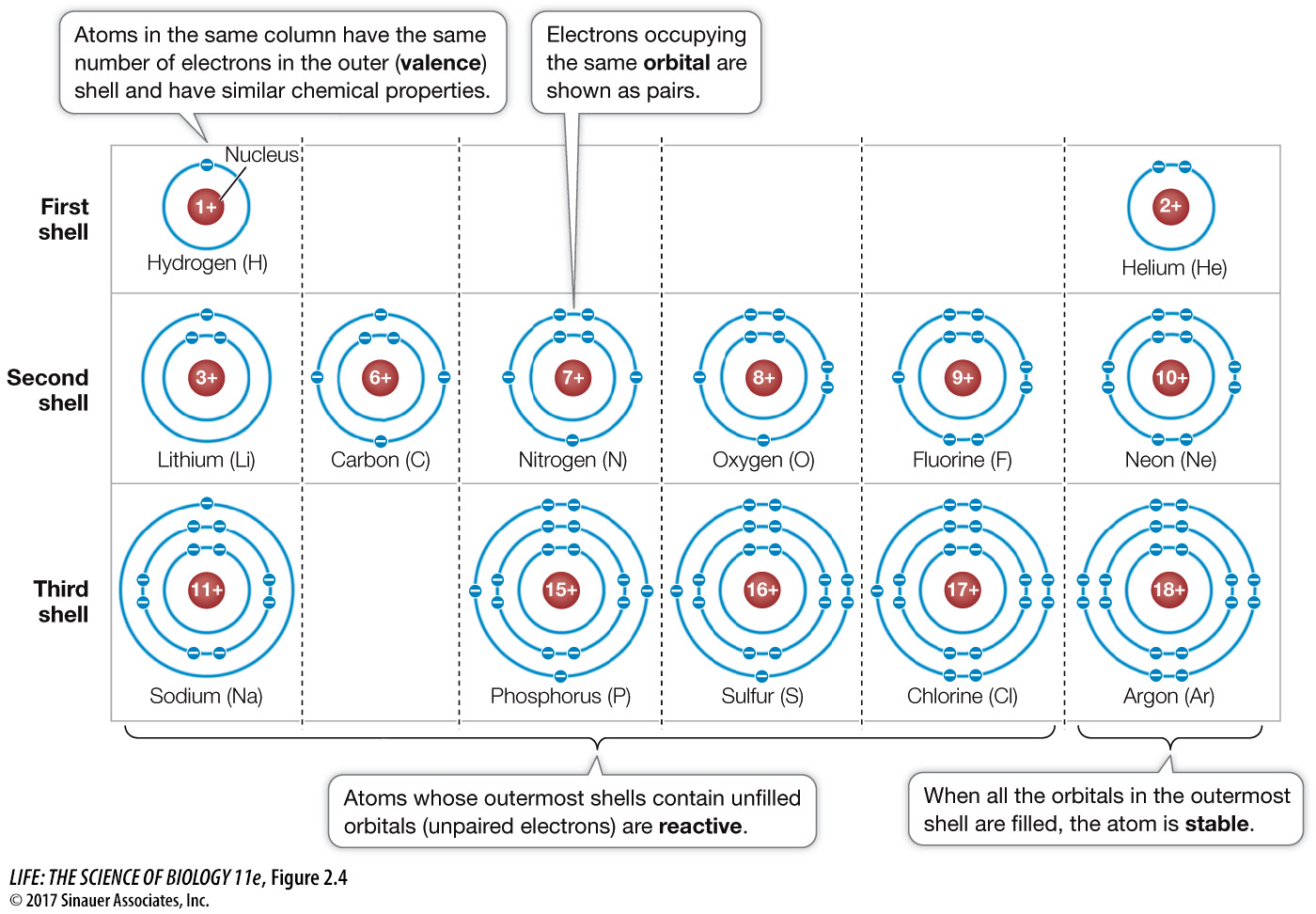
Figure 2.4 Electron Shells Determine the Reactivity of Atoms Each shell can hold a specific maximum number of electrons. Going out from the nucleus, each shell must be filled before electrons can occupy the next shell. The energy level of an electron is higher in a shell farther from the nucleus. An atom with unpaired electrons in its outermost shell can react (bond) with other atoms. Note that the atoms in this figure are arranged similarly to their arrangement in the periodic table.