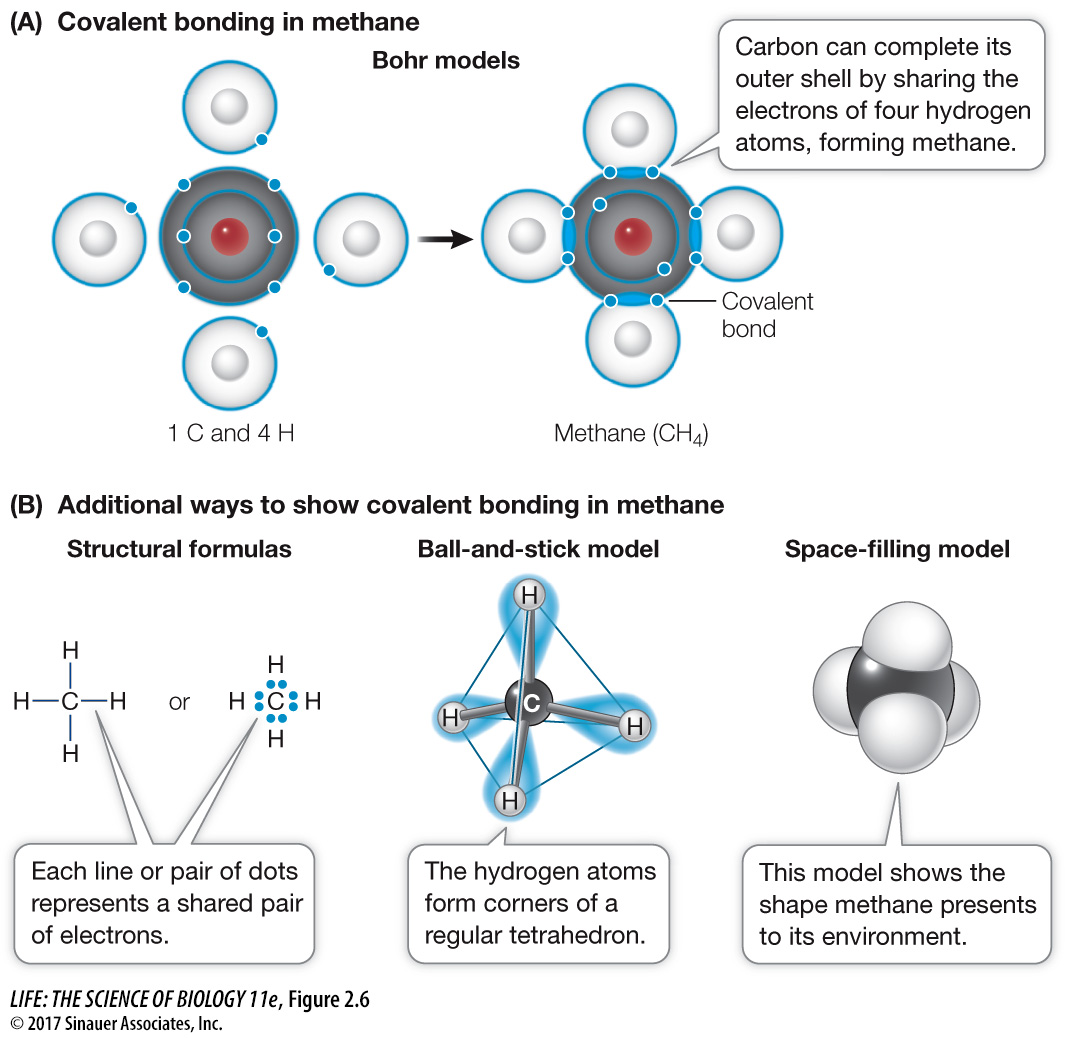
Figure 2.6 Covalent Bonding Can Form Compounds (A) Bohr models showing the formation of covalent bonds in methane, whose molecular formula is CH4. Electrons are shown in shells around the nucleus. (B) Three additional ways of representing the structure of methane. In a structural formula, a covalent bond can be indicated with a single line or a shared pair of electron dots. The ball- d- e- e-