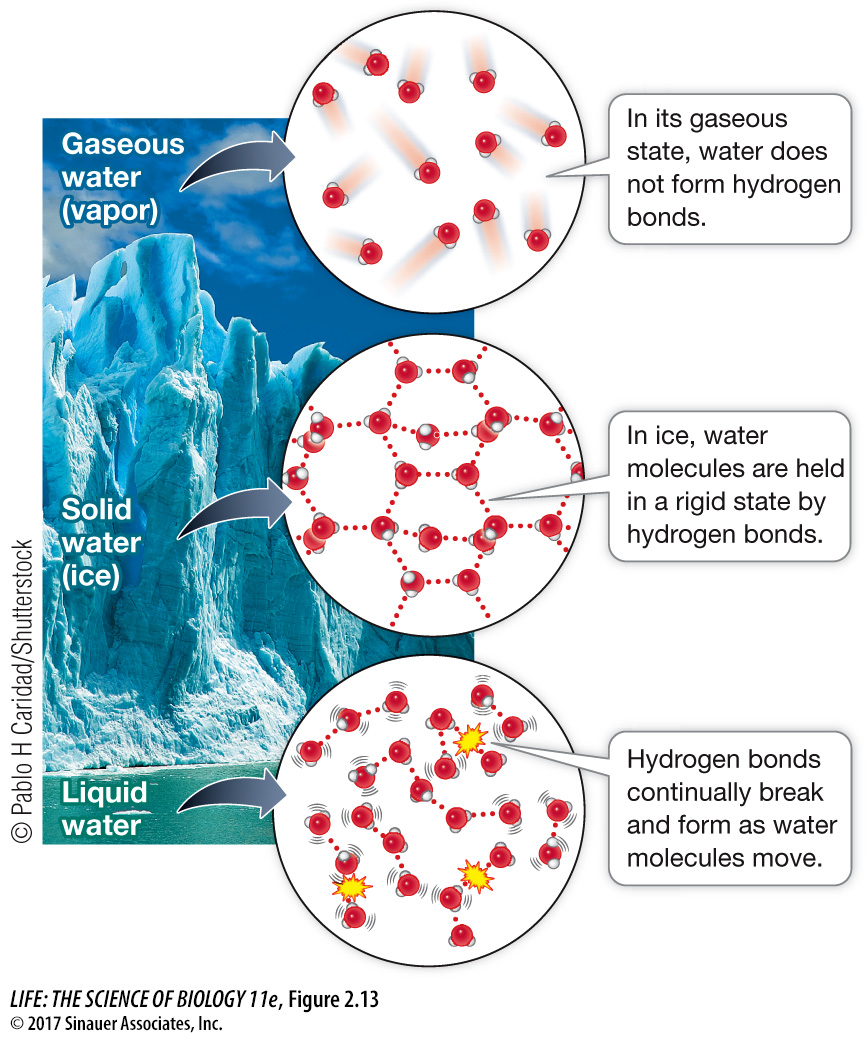
Figure 2.13 Hydrogen Bonding and the Properties of Water Hydrogen bonding occurs between the molecules of water in both its liquid and solid states. Ice is more structured but less dense than liquid water, which is why ice floats. Water forms a gas when its hydrogen bonds are broken and the molecules move farther apart.