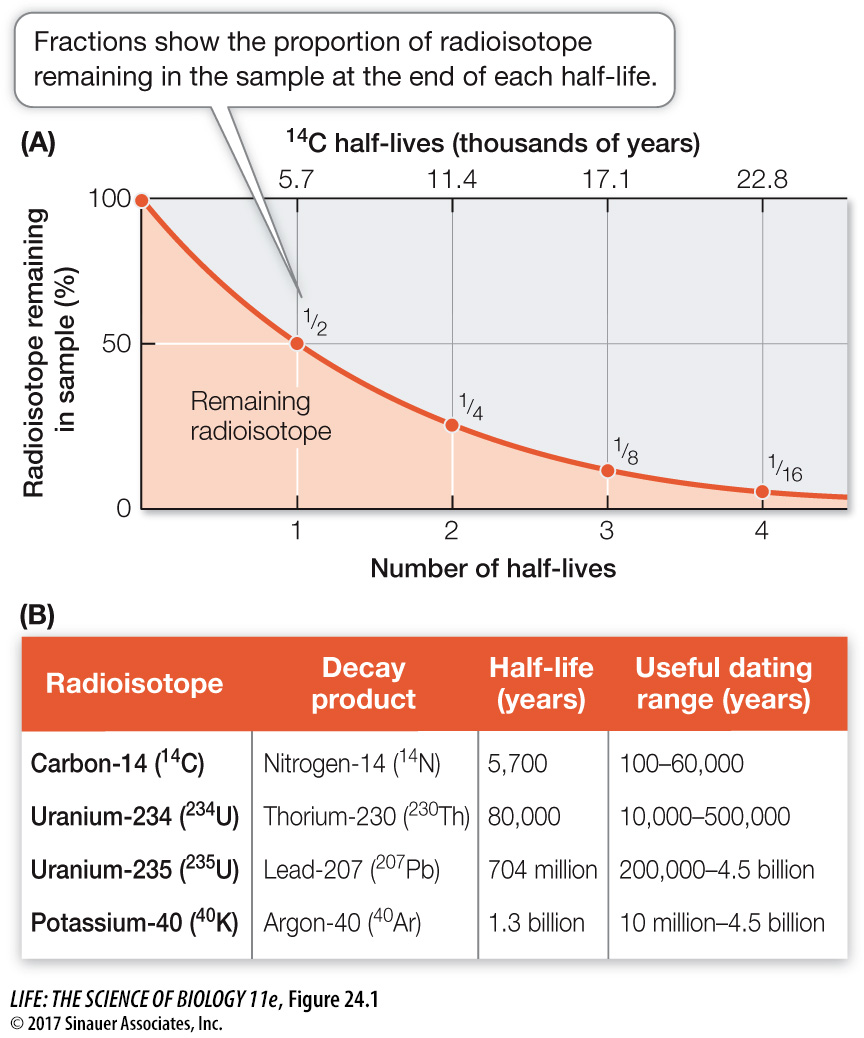
Figure 24.1 Radioactive Isotopes Allow Us to Date Ancient Rocks The decay of radioactive isotopes into stable isotopes happens at a steady rate. A half- life is the time it takes for half of the remaining atoms to decay in this way. (A) The graph demonstrates the principle of half- life using carbon- 14 (14C) as an example. The half- life of 14C is 5,700 years. (B) Different radioisotopes have different characteristic half- lives that allow us to estimate the ages of many rocks.