Ionic attractions form by electrical attraction
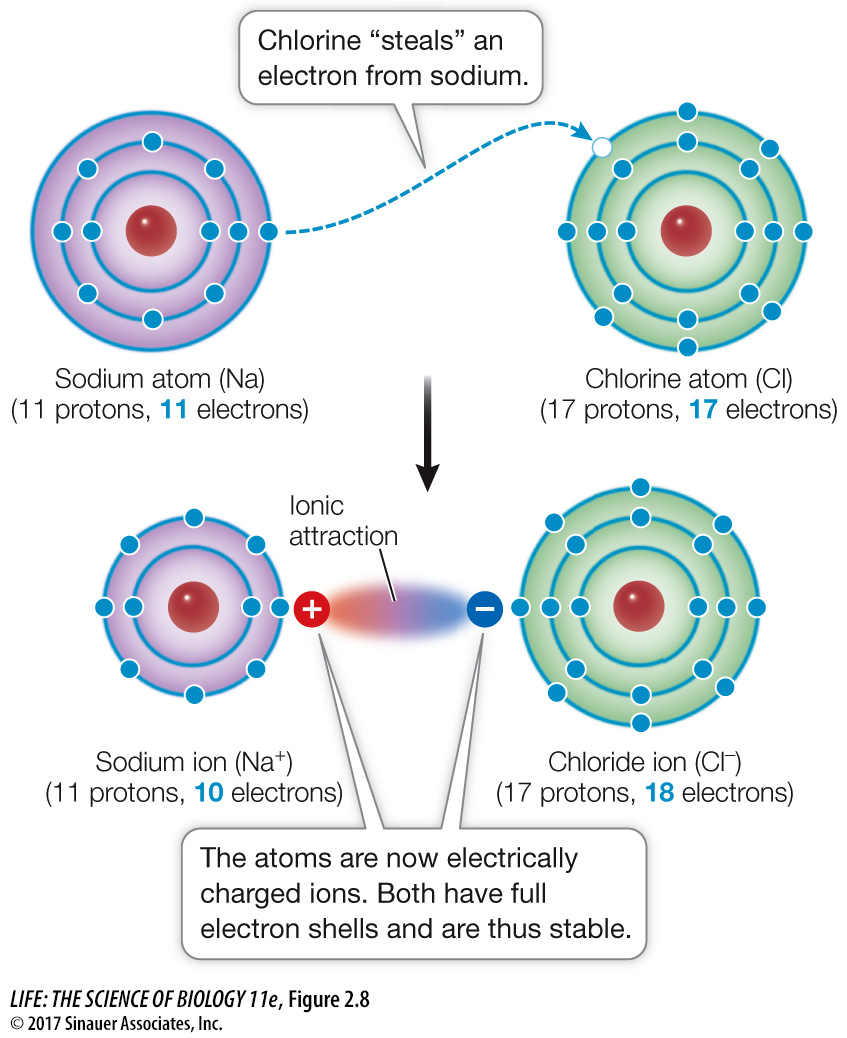
When one interacting atom is much more electronegative than the other, a complete transfer of one or more electrons may take place. Consider sodium (electronegativity 0.9) and chlorine (electronegativity 3.1). A sodium atom has only one electron in its outermost shell; this condition is unstable. A chlorine atom has seven electrons in its outermost shell—
Question
Q: What ions are formed when calcium ion reacts with chlorine? See the periodic table in Figure 2.2.
Ca (atomic number 20) has two valence electrons. Cl (atomic number 17) has seven valence electrons. So combining, two electrons are lost from one Ca atom to two atoms of Cl: formula CaCl2.
Ions are electrically charged particles that form when atoms gain or lose one or more electrons:
The sodium ion (Na+) in our example has a +1 unit of charge because it has one less electron than it has protons. The outermost electron shell of the sodium ion is full, with eight electrons, so the ion is stable. Positively charged ions are called cations. Other biologically important cations include Ca2+, H+, Mg2+, and K+.
The chloride ion (Cl–) has a –1 unit of charge because it has one more electron than it has protons. This additional electron gives Cl– a stable outermost shell with eight electrons. Negatively charged ions are called anions.
Some elements can form ions with multiple charges by losing or gaining more than one electron. Examples are Ca2+ (the calcium ion, a calcium atom that has lost two electrons) and Mg2+ (the magnesium ion). Two biologically important elements can each yield more than one stable ion. Iron yields Fe2+ (the ferrous ion) and Fe3+ (the ferric ion), and copper yields Cu+ (the cuprous ion) and Cu2+ (the cupric ion). Groups of covalently bonded atoms that carry an electric charge are called complex ions; examples include NH4+ (the ammonium ion), SO42–
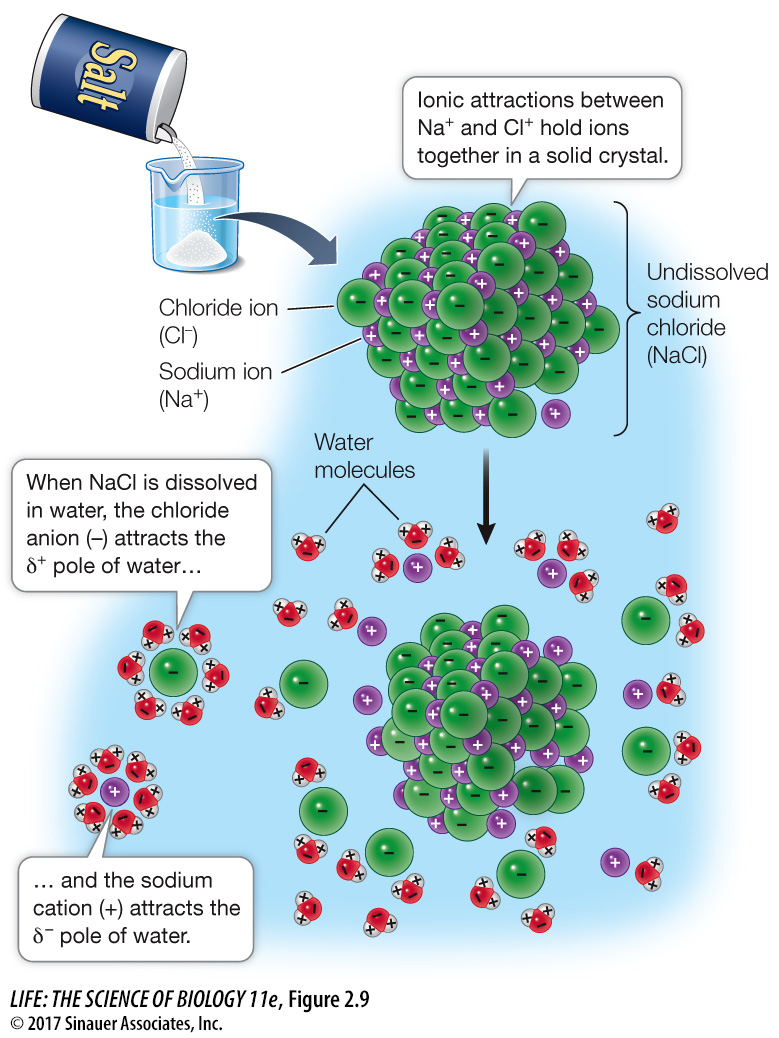
Ionic attractions are bonds formed as a result of the electrical attraction between ions bearing opposite charges. Ions can form bonds that result in stable solid compounds, which we call salts. You’re familiar with sodium chloride (NaCl)—table salt; its cations (Na+) and anions (Cl–) are held together by ionic attractions. In solids, the attractions are strong because the ions are close together. However, when ions are dispersed in water, the distances between them can be large, greatly reducing the strength of the attraction. Under the conditions in living cells, an ionic attraction is less strong than a covalent bond (see Table 2.1).
Not surprisingly, ions can interact with polar molecules, since both are charged. This interaction results when a solid salt such as NaCl dissolves in water. Water molecules surround the individual ions, separating them (Figure 2.9). The negatively charged chloride ions attract the positive poles of the water molecules, while the positively charged sodium ions attract the negative poles of the water molecules. This special property of water (its polarity) is one reason it is such a good biological solvent (see Key Concept 2.4).
Question
Q: What happens at the chemical and physical levels when a salt solution evaporates?
At the chemical level, evaporation eliminates water for hydration of the ions, so the ions are no longer separated. At the physical level, the salt no longer dissolves and crystals form.