key concept2.3Atoms Change Partners in Chemical Reactions
A hallmark of life is that it is dynamic. Things don’t sit still, especially at the chemical level. Atoms bonded to other atoms can leave a binding relationship and find a new partner. Bear in mind that this does not happen all the time: your skin is a biological tissue, and its atoms form a stable structure. But change is common, especially among the atoms and molecules dissolved in water. A chemical reaction occurs when moving atoms collide with sufficient energy to combine or to change their bonding partners.
focus your learning
Chemical reactions obey the laws of conservation of energy and conservation of matter.
Chemical reactions are accompanied by changes in energy.
Consider the combustion reaction that takes place in the flame of a propane stove. When propane (C3H8) reacts with oxygen gas (O2), the carbon atoms become bonded to oxygen atoms instead of hydrogen atoms, and the hydrogen atoms become bonded to oxygen instead of carbon (Figure 2.12). As the covalently bonded atoms change partners, the composition of the matter changes; propane and oxygen gas become carbon dioxide and water. This chemical reaction can be represented by the equation
C3H8 + 5 O2 → 3 CO2 + 4 H2O + Energy
Reactants → Products
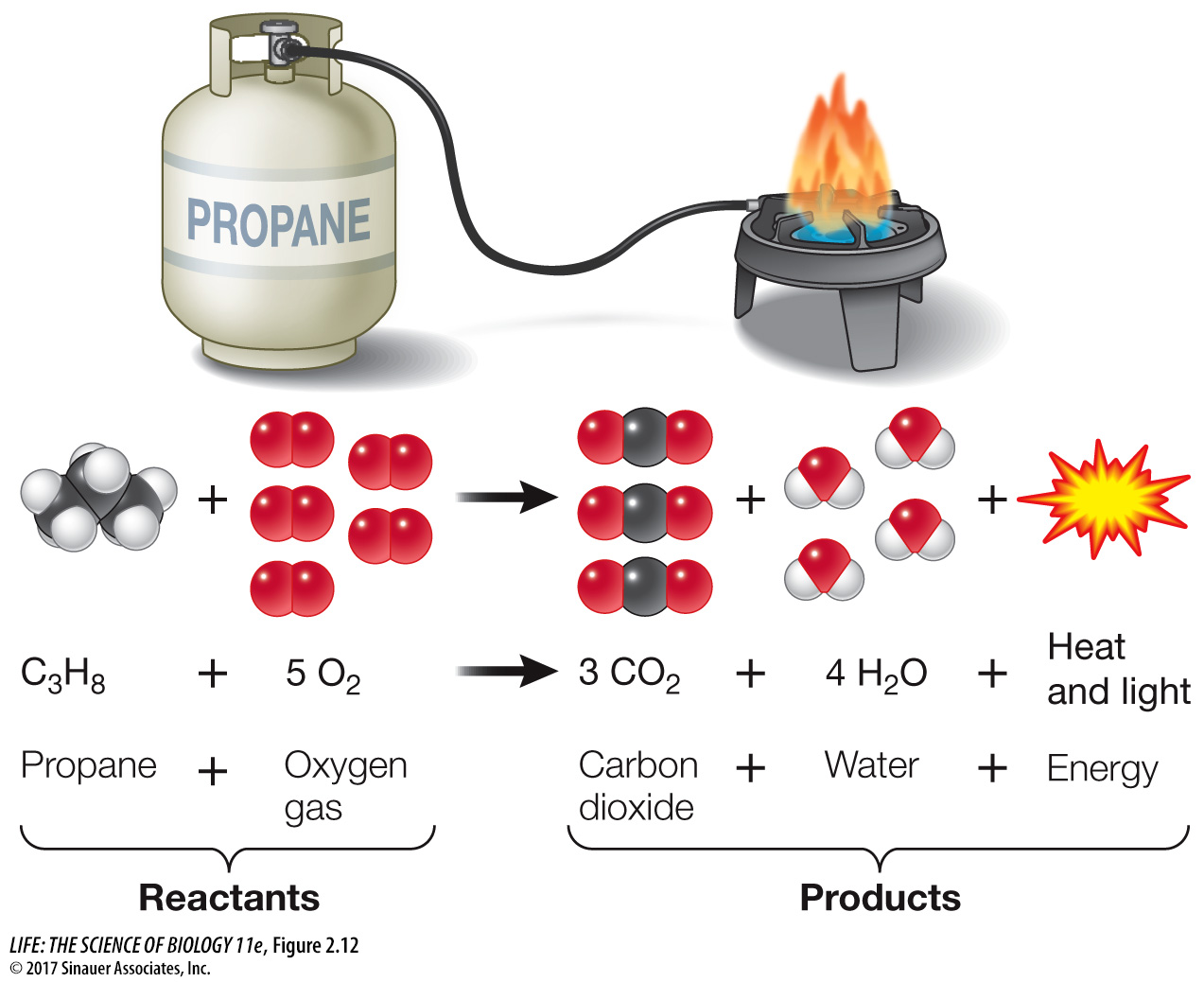
In this equation, propane and oxygen are the reactants, and carbon dioxide and water are the products. This example is an oxidation-
34
The electron acceptor, or oxidizing agent, gains electrons and is said to be reduced in a chemical reaction.
The electron donor, or reducing agent, loses electrons and is said to be is oxidized in a chemical reaction.
Can you identify the oxidizing and reducing agents in our example? Because electrons and protons (i.e., hydrogen atoms) are transferred from propane, propane is the reducing agent and oxygen is the oxidizing agent, which in this example forms water upon accepting the hydrogens. You will see many examples of redox reactions involving electron/proton transfer in later chapters.
The products of a chemical reaction can have very different properties from the reactants. The reaction shown in Figure 2.12 is said to be complete: all the propane and oxygen are used up in forming the two products. The arrow symbolizes the direction of the chemical reaction. The numbers preceding the molecular formulas indicate how many molecules are used or produced.
Note that in this and all other chemical reactions, matter is neither created nor destroyed. The total number of carbon atoms on the left side of the equation (3) equals the total number of carbon atoms on the right (3). In other words, the equation is balanced. However, there is another aspect of this reaction: the heat and light of the stove’s flame reveal that the reaction between propane and oxygen releases a great deal of energy.
Energy is defined as the capacity to do work, but in the context of chemical reactions, it can be thought of as the capacity for change. Chemical reactions do not create or destroy energy, but changes in the form of energy usually accompany chemical reactions.
In the reaction between propane and oxygen, a large amount of heat energy is released. This energy was present in another form, called potential chemical energy, in the covalent bonds within the propane and oxygen gas molecules. Not all reactions release energy; indeed, many chemical reactions require that energy be supplied from the environment. Some of this energy is then stored as potential chemical energy in the bonds formed in the products. We will see in future chapters how reactions that release energy and reactions that require energy are often coupled.
Many chemical reactions take place in living cells, and some of these have a lot in common with the oxidation–