Water has a unique structure and special properties
The molecule H2O has unique chemical features. As you have already learned, water is a polar molecule that can form hydrogen bonds. The four pairs of electrons in the outer shell of the oxygen atom repel one another, giving the water molecule a tetrahedral shape:
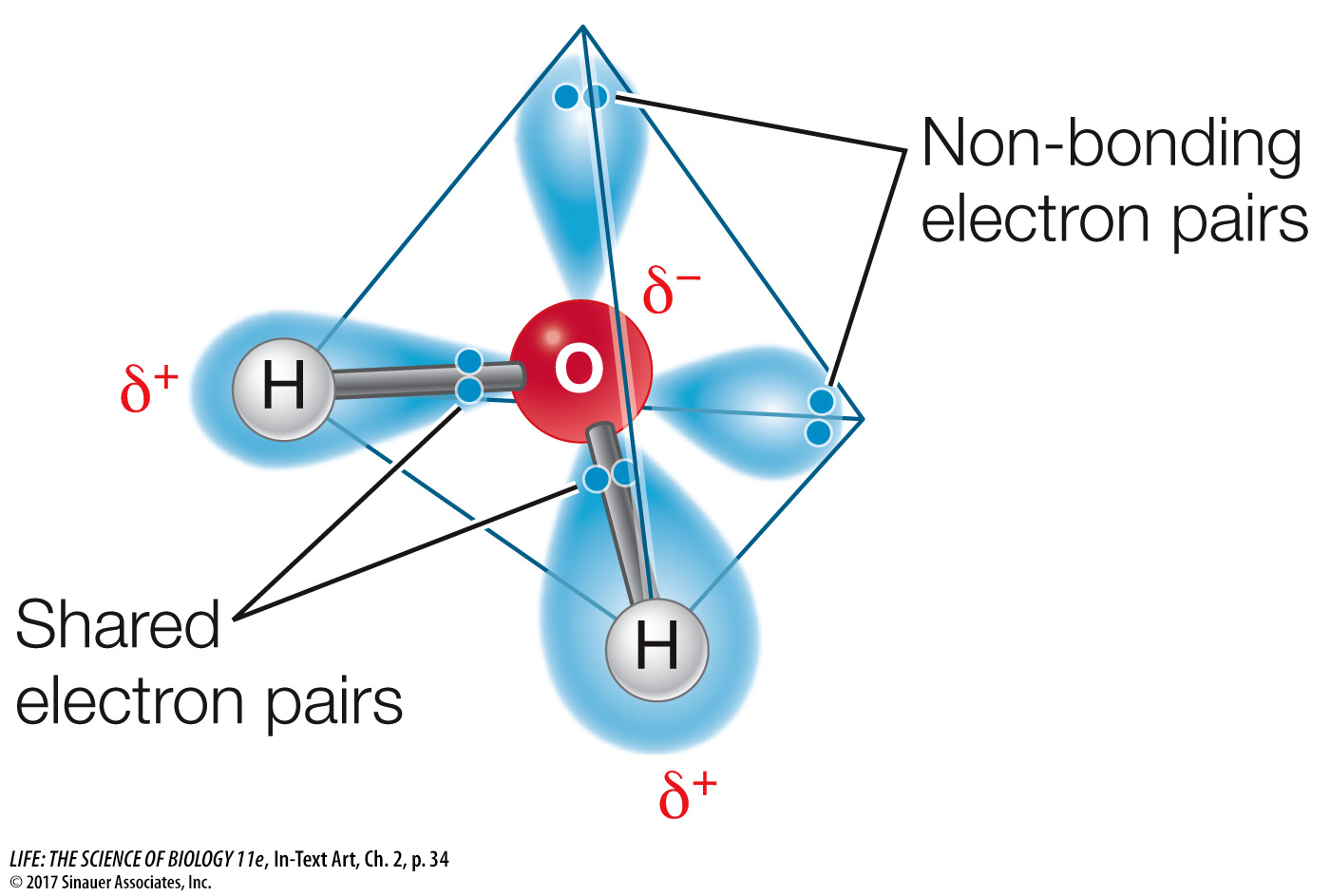
The chemical features of water explain some of its interesting properties, such as the ability of ice to float, the melting and freezing temperatures of water, the ability of water to store heat, the formation of water droplets, water’s ability to dissolve many substances, and its inability to dissolve many others.
35
ICE FLOATS In water’s solid state (ice), individual water molecules are held in place by hydrogen bonds. Each molecule is bonded to four other molecules in a rigid, crystalline structure (Figure 2.13). Although the molecules are held firmly in place, they are farther apart from one another than they are in liquid water, where the molecules are moving about. In ice, there are cavities between individual water molecules. In other words, solid water is less dense than liquid water, which is why ice floats.
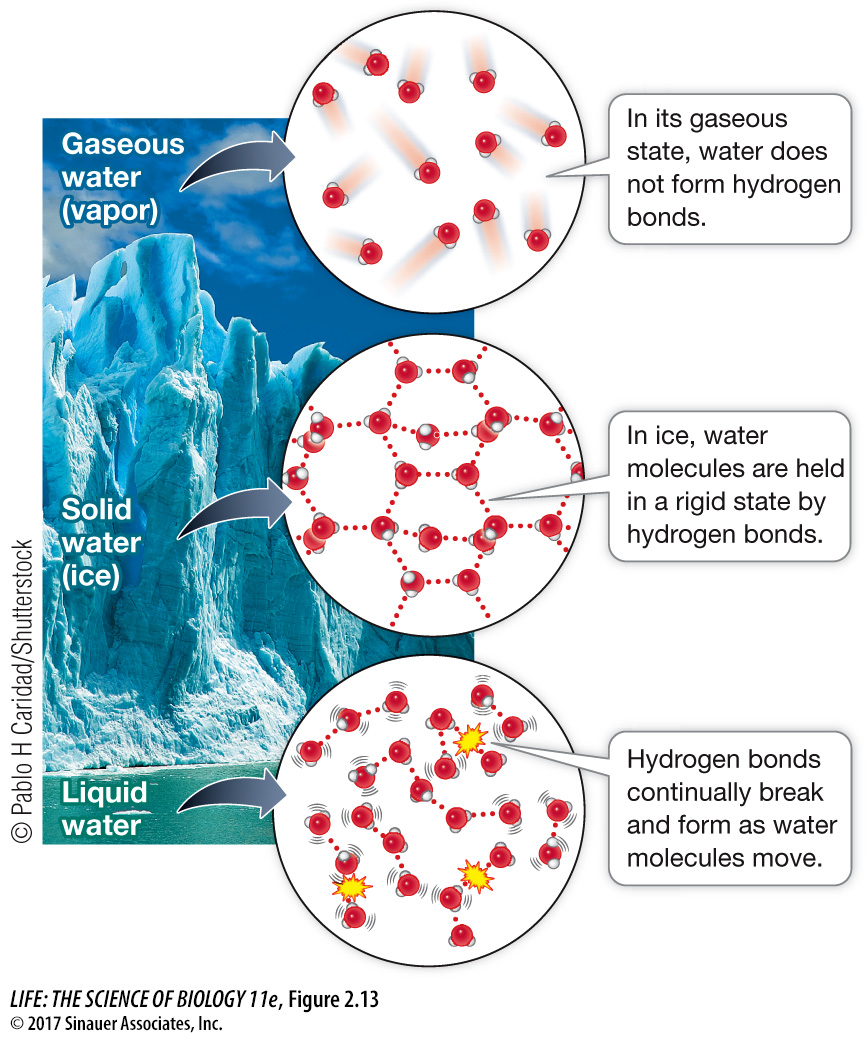
Think of the biological consequences if ice were to sink in water. A pond would freeze from the bottom up, becoming a solid block of ice in winter and killing most of the organisms living there. Once the whole pond was frozen, its temperature could drop well below the freezing point of water. But because ice floats, it forms an insulating layer on the top of the pond, and reduces heat flow to the cold air above. Thus fish, plants, and other organisms in the pond are not subjected to temperatures lower than 0°C, which is the freezing point of pure water.
MELTING, FREEZING, AND HEAT CAPACITY Compared with many other substances that have molecules of similar size, ice requires a great deal of heat energy to melt. The amount of heat energy required to raise the temperature of 1 gram of a substance by 1°C is called its specific heat. Water has a relatively high specific heat because so many hydrogen bonds connecting the water molecules in ice must be broken to change water from solid to liquid. In the opposite process—
Because it takes a lot of energy to change the physical state of water, water temperatures in oceans and other large bodies of water are remarkably constant throughout the year. The temperature changes of coastal land masses are in turn moderated by large bodies of water. Indeed, water helps minimize variations in atmospheric temperature across the planet. Water’s ability to moderate heat helps it function as an insulator, helping prevent an organism’s body temperature from rising during a sunny day.
Water also has a high heat of vaporization, which means that a lot of heat is required to change water from its liquid to its gaseous state (the process of evaporation). Once again, much of the heat energy is used to break the many hydrogen bonds between the water molecules. This heat must be absorbed from the environment in contact with the water. *Evaporation thus has a cooling effect on the environment—

*connect the concepts Living systems use the evaporation of water, which disrupts hydrogen bonds, to dissipate excess heat that would otherwise cause problems. See Key Concept 38.3 for examples in plants, and Key Concepts 39.3–39.5 for examples in animals.
COHESION AND SURFACE TENSION In liquid water, individual molecules are able to move about. The hydrogen bonds between the molecules continually form and break (see Figure 2.14). Chemists estimate that this occurs about a trillion times a minute for a single water molecule!
At any given time, a water molecule forms on average 3.4 hydrogen bonds with other water molecules. These hydrogen bonds explain the cohesive strength of liquid water. This cohesive strength, or cohesion, is defined as the capacity of water molecules to resist coming apart from one another when placed under tension. Water’s *cohesive strength permits narrow columns of liquid water to move from the roots to the leaves of tall trees. When water evaporates from the leaves, the entire column moves upward in response to the pull of the molecules at the top (Figure 2.14B). A related property is adhesion, the attraction of water molecules to other molecules of a different type. For example, when you put a straw into a cup with water, it “climbs” up the straw so the column is higher than the level in the cup. This adhesive behavior of water—
*connect the concepts As described in Key Concept 34.2, the transpiration–
36
The surface of liquid water exposed to the air is difficult to puncture because the water molecules at the surface are hydrogen-