Aqueous solutions may be acidic or basic
When some substances dissolve in water, they release hydrogen ions (H+), which are actually single, positively charged protons. Hydrogen ions can interact with other molecules and change their properties. For example, the protons in “acid rain” can damage plants, and you probably have experienced the excess of hydrogen ions that we call “acid indigestion.”
Here we will examine the properties of acids (defined as substances that release H+) and bases (defined as substances that accept H+). We will distinguish between strong and weak acids and bases, and provide a quantitative means for stating the concentration of H+ in solutions: the pH scale.
37
ACIDS RELEASE H+ When hydrochloric acid (HCl) is added to water, it dissolves, releasing the ions H+ and Cl–:
HCl → H+ + Cl–
Because its H+ concentration has increased, the solution is acidic.
Acids are substances that release H+ ions in solution. HCl is an acid, as is H2SO4 (sulfuric acid). One molecule of sulfuric acid will ionize to yield two H+ and one SO42–
—COOH → —COO– + H+
Acids that fully ionize in solution, such as HCl and H2SO4, are called strong acids. However, not all acids ionize fully in water. For example, if acetic acid (CH3COOH) is added to water, some of it will dissociate into two ions (CH3COO– and H+), but some of the original acetic acid will remain as well. Because the reaction is not complete, acetic acid is a weak acid.
BASES ACCEPT H+ Bases are substances that accept H+ in solution. As with acids, there are strong and weak bases. If NaOH (sodium hydroxide) is added to water, it dissolves and ionizes, releasing OH– and Na+ ions:
NaOH → Na+ + OH–
Because OH– absorbs H+ to form water, such a solution is basic. This reaction is complete, and so NaOH is a strong base.
Weak bases include the bicarbonate ion (HCO3–), which can accept an H+ ion and become carbonic acid (H2CO3), and ammonia (NH3), which can accept H+ and become an ammonium ion (NH4+). Biological compounds that contain —NH2 (the amino group) are also bases because —NH2 accepts H+:
—NH2 + H+ → —NH3+
ACID–
CH3COOH → CH3COO– + H+
Then, once the ions are formed, some of them re-
CH3COO– + H+ → CH3COOH
This pair of reactions is reversible. A reversible reaction can proceed in either direction—
CH3COOH ~ CH3COO– + H+
In terms of acids and bases, there are two types of reactions, depending on the extent of the reversibility:
The ionization of strong acids and bases in water is virtually irreversible.
The ionization of weak acids and bases in water is somewhat reversible.
WATER IS A WEAK ACID AND A WEAK BASE The water molecule has a slight but significant tendency to ionize into a hydroxide ion (OH–) and a hydrogen ion (H+). Actually, two water molecules participate in this reaction. One of the two molecules “captures” a hydrogen ion from the other molecule, forming a hydroxide ion and a hydronium ion:
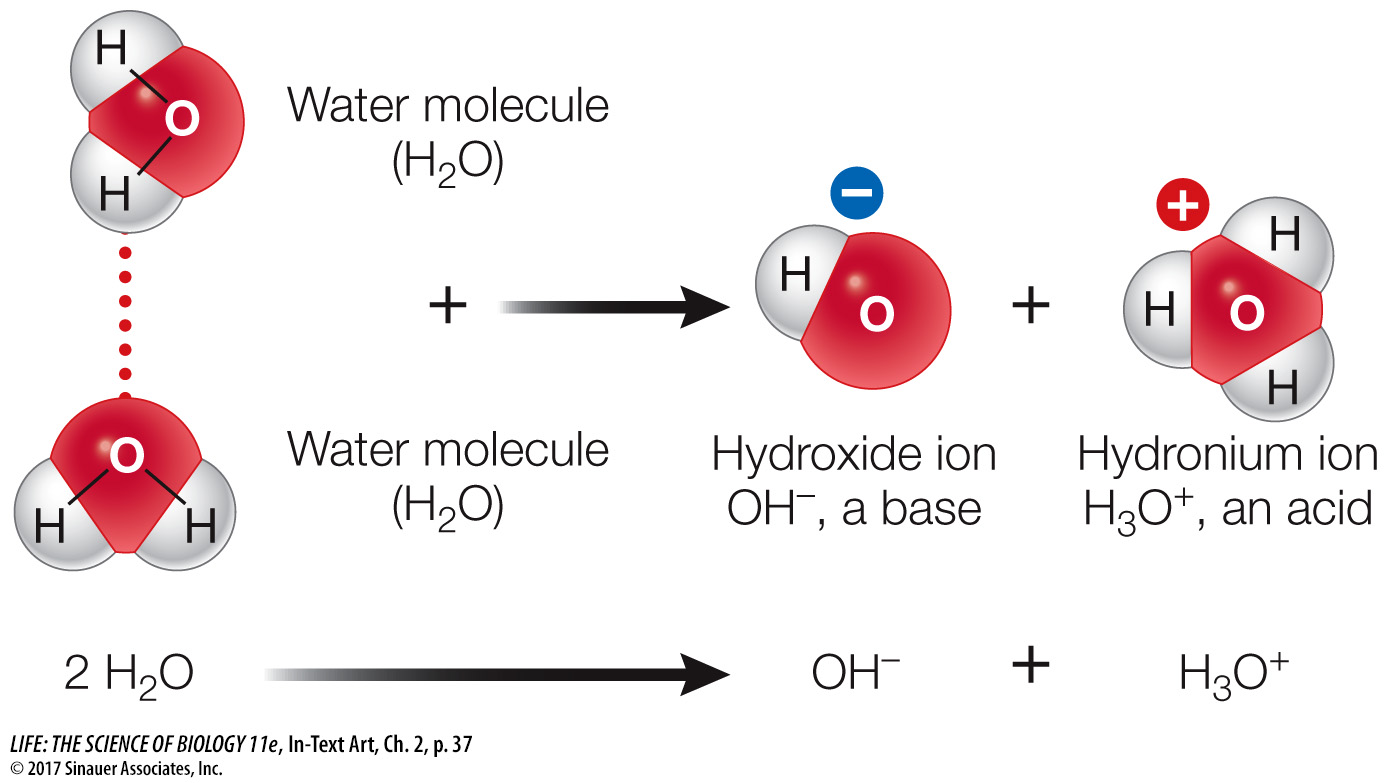
The hydronium ion is, in effect, a hydrogen ion bound to a water molecule. For simplicity, biochemists tend to use a modified representation of the ionization of water:
H2O → H+ + OH–
The ionization of water is important to all living creatures. This fact may seem surprising, since only about 1 water molecule in 500 million is ionized at any given time. But this is less surprising if we focus on the abundance of water in living systems, and the reactive nature of the H+ ions produced by ionization.
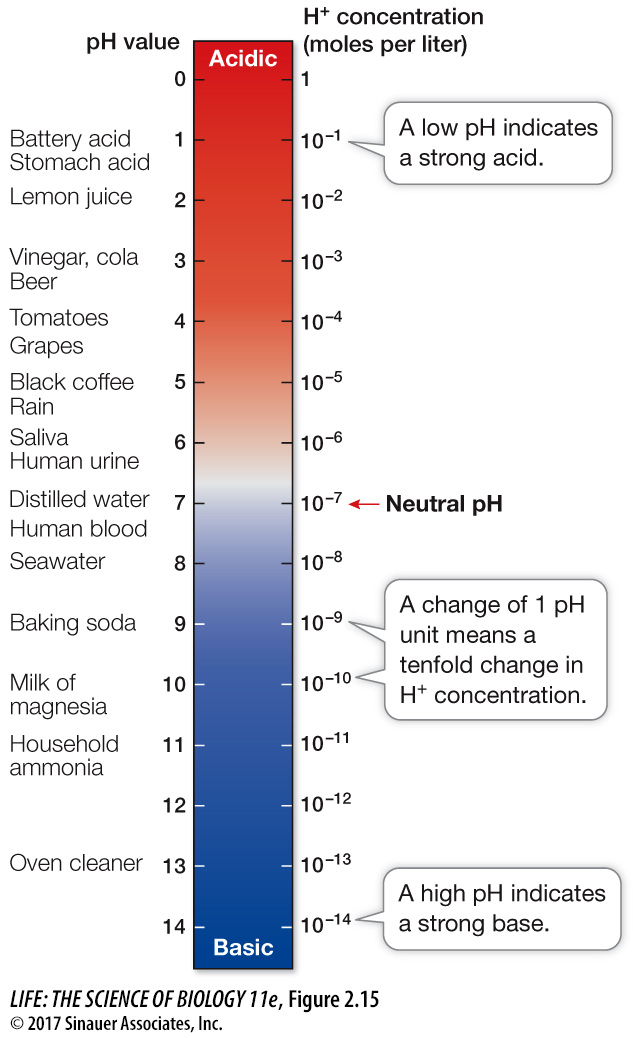
pH: HYDROGEN ION CONCENTRATION As we have seen, compounds can be either acids or bases, and thus solutions can be either acidic or basic. We can measure how acidic or basic a solution is by measuring its concentration of H+ in moles per liter (its molarity). Here are some examples:
Pure water has a H+ concentration of 10–7 M.
A 1 M HCl solution has a H+ concentration of 1 M (recall that all the HCl dissociates into its ions).
A 1 M NaOH solution has a H+ concentration of 10–14 M.
This is a very wide range of numbers to work with—
pH = −log(H+) where (H+) is the concentration of free H+ in solution
Since the H+ concentration of pure water is 10–7 M, its pH is –log(10–7) = –(–7), or 7. A smaller negative logarithm means a larger number. In practical terms, a lower pH means a higher H+ concentration, or greater acidity. In 1 M HCl, the H+ concentration is 1 M, so the pH is the negative logarithm of 1 (–log 100), or 0. The pH of 1 M NaOH is the negative logarithm of 10–14, or 14.
A solution with a pH of less than 7 is acidic—
38
Why is this discussion of pH so relevant to biology? Many reactions involve the transfer of an ion or charged group from one molecule to another, and the presence of positive or negative ions in the environment can greatly influence the rates of such reactions. Furthermore, pH can influence the shapes of molecules. Many biologically important molecules contain charged groups (e.g., —COO–) that can interact with the polar regions of water, and these interactions influence the way such molecules fold up into three-
BUFFERS The maintenance of internal constancy—
A buffer is a mixture of a weak acid and its corresponding base, or a weak base and its corresponding acid. For example, a weak acid is carbonic acid (H2CO3), and its corresponding base is the bicarbonate ion (HCO3–). If another acid is added to a solution containing this mixture (a buffered solution), not all the H+ ions from the acid remain in solution. Instead, many of them combine with the bicarbonate ions to produce more carbonic acid:
HCO3– + H+ ⇌ H2CO3
This reaction uses up some of the H+ ions in the solution and decreases the acidifying effect of the added acid. If a base is added, the reaction essentially reverses. Some of the carbonic acid ionizes to produce bicarbonate ions and more H+, which counteracts some of the added base. The buffer minimizes the effect that an added acid or base has on pH. The carbonic acid/bicarbonate buffering system is present in the blood, where it is important for preventing significant changes in pH that could disrupt the ability of the blood to carry vital oxygen to tissues. A given amount of acid or base causes a smaller pH change in a buffered solution than in a non-
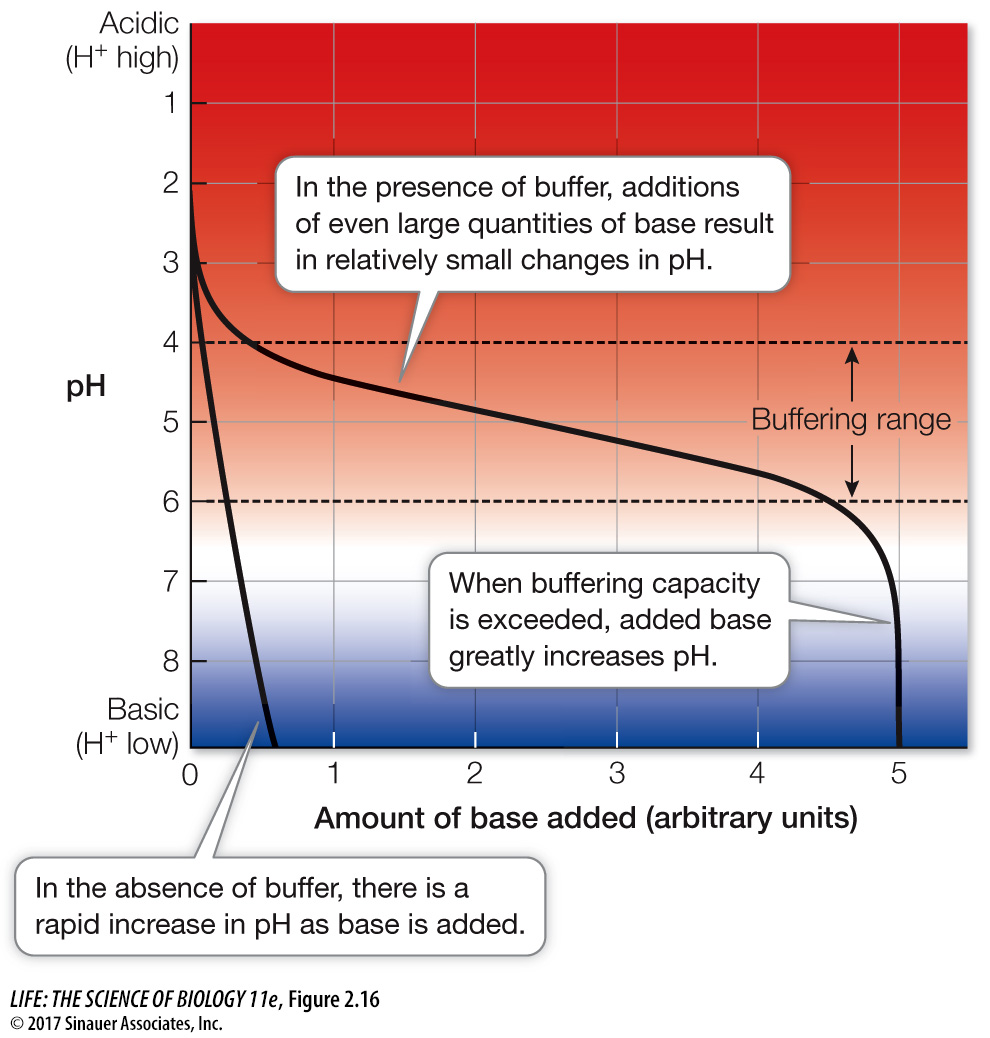
Buffers illustrate an important chemical principle of reversible reactions, called the law of mass action. Addition of a reactant on one side of a reversible system drives the reaction in the direction that uses up that compound. In the case of buffers, addition of an acid drives the reaction in one direction; addition of a base drives the reaction in the other direction.
You may occasionally use a buffer to relieve indigestion. The lining of the stomach constantly secretes hydrochloric acid, making the stomach contents acidic. But excessive stomach acid can cause discomfort. We can relieve this discomfort by ingesting a salt such as NaHCO3 (sodium bicarbonate), which acts as a buffer.