Apply What You’ve Learned
Review
2.4 The acid–
Scientists are developing methods to sample a person’s breath and test it for higher-
Insulin is a hormone that stimulates cells in the body to take up glucose from the blood. Cells use glucose as a source of energy for normal cell function. In type I diabetes, insulin is not produced, and glucose does not enter cells from the blood. Lacking an incoming supply of glucose, cells begin breaking down fats as a source of energy. When fats break down, several ketone-
One of the ketone-
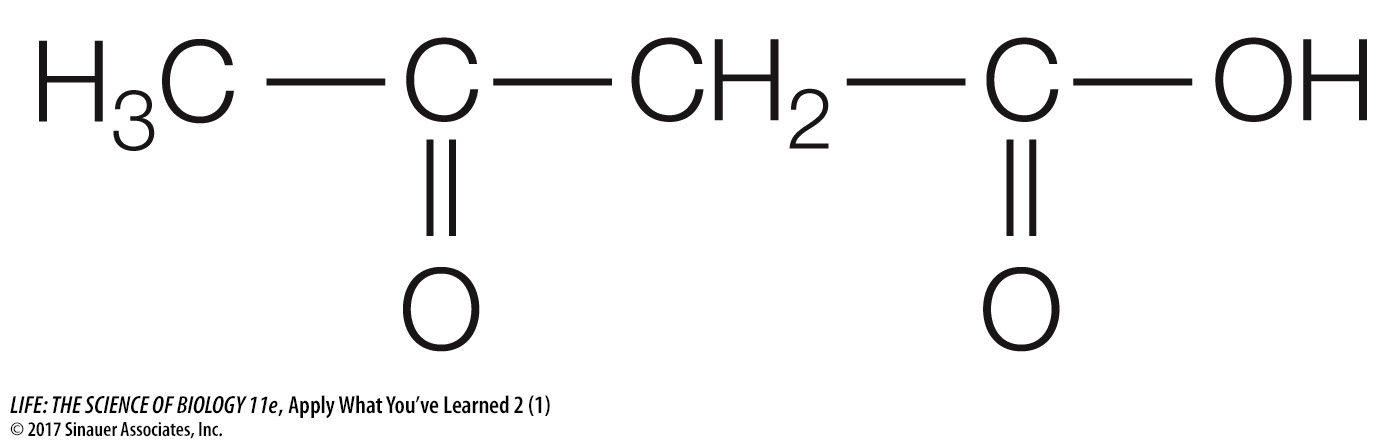
Questions
Question 1
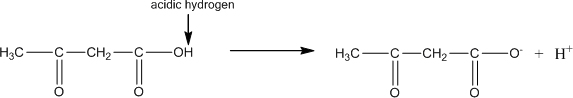
In water, acetoacetic acid can ionize to form protons and acetoacetate ions. Write the chemical structures for this reaction.
Question 2
Now write out the reversible chemical reaction for the ionization of carbonic acid in blood, which can act as a buffer. Would the pH of blood change if a small amount of acetoacetic acid produced normally in tissues were added to the blood? Explain.
HCO3– + H+ ⇌ H2CO3
The pH would not change. As H+ was added due to ionization of acetoacetic acid, it would push the equilibrium of the above reaction to the right, as shown below.
HCO3– + H+ → H2CO3
added H+ reacts with HCO3– to shift equilibrium to the right
That is, bicarbonate ions would attach to the added H+, so the overall free H+ would not change.
Question 3
What would happen to the pH of the blood if more and more acetoacetic acid built up, as in a case of untreated diabetes? Explain, using what you found in Question 2 above. What would happen to the body’s ability to maintain a constant blood pH? How does this explain why people with this condition become severely ill?
Eventually the bicarbonate buffer system would be overwhelmed. It would no longer be able to take up the additional H+ ions building up in the blood and shuttle them into carbonic acid. At that point, H+ ions would accumulate, which would cause the blood pH to drop. The body would not be able to maintain a constant pH and its cells would be exposed to lower than normal pH conditions in the blood. In this case, the person would become severely ill because the cells would be exposed to an abnormal environment that then disrupts normal cell functions.
Question 4
Acetone is one of the compounds produced from fat breakdown and expelled in the breath. It is targeted for detection by a breath-
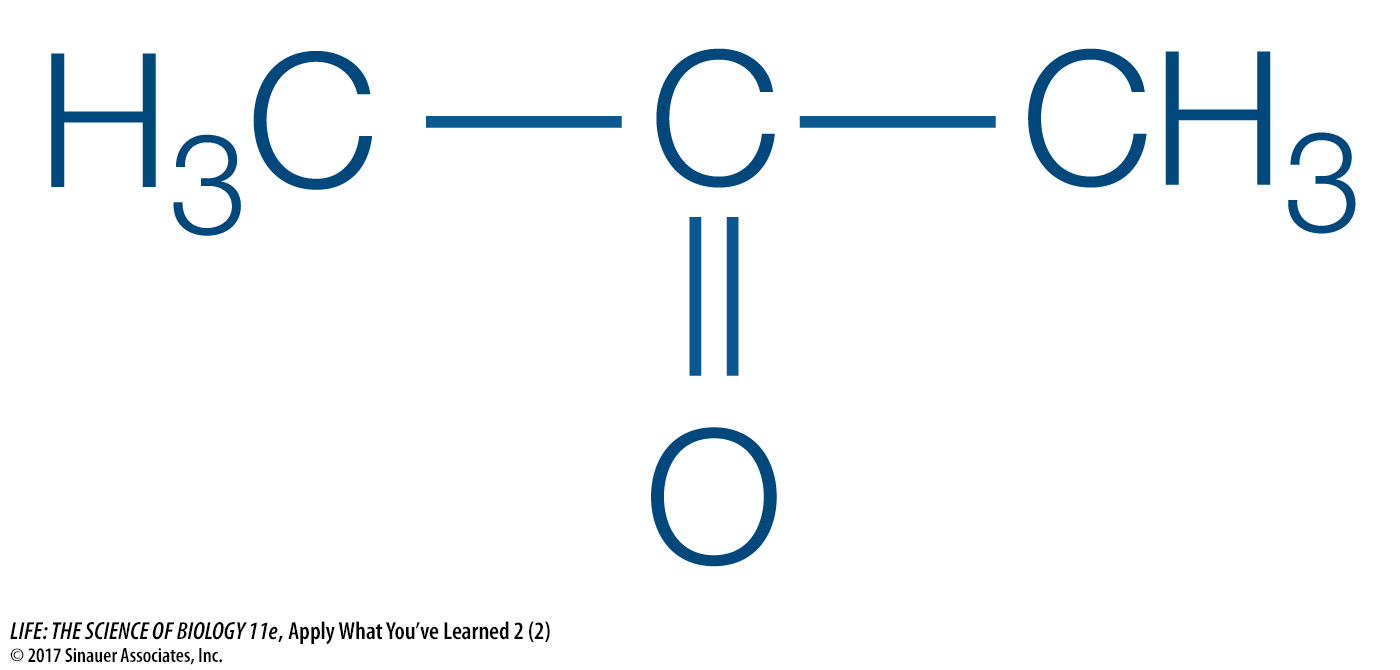
Acetone does not contain an acidic hydrogen, so it would not be expected to affect any acid or base buffering systems in the blood.
Question 5
Suppose scientists succeed in developing a device that detects ketone buildup in the breath. If a person tested positive for higher-
Four test possibilities are: insulin level (lower than normal), blood glucose level (elevated), blood pH (lower than normal), and bicarbonate level (lower than normal).