Each element has a unique number of protons
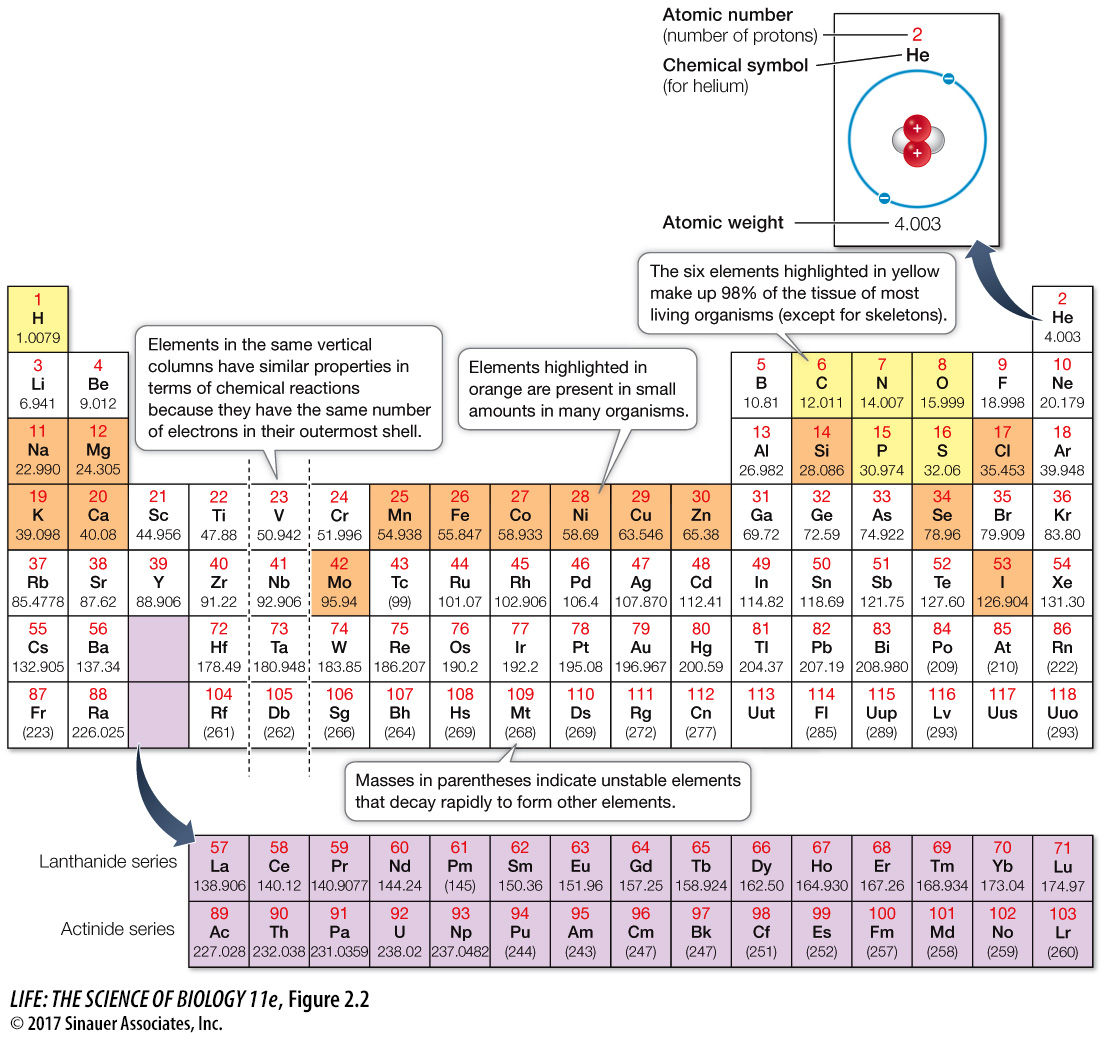
An element differs from other elements by the number of protons in the nucleus of each of its atoms; the number of protons is designated the atomic number. This atomic number is unique to each element and does not change. The atomic number of helium is 2, and an atom of helium always has two protons; the atomic number of oxygen is 8, and an atom of oxygen always has eight protons. Since the number of protons (and electrons) determines how an element behaves in chemical reactions, it is possible to arrange the elements in a table such that those with similar chemical properties are grouped together. This is the familiar periodic table you see in Figure 2.2.
Media Clip 2.1 The Elements Song
www.life11e.com/
Along with a definitive number of protons, every element except hydrogen has one or more neutrons in its nucleus. The mass number of an atom is the total number of protons and neutrons in its nucleus. The nucleus of a carbon atom contains six protons and six neutrons and has a mass number of 12. Oxygen has eight protons and eight neutrons and has a mass number of 16. Since the mass of an electron is negligible, the mass number is essentially the mass of the atom in daltons.
By convention, we often print the symbol for an element with the atomic number at the lower left and the mass number at the upper left, both immediately preceding the symbol. Thus hydrogen, carbon, and oxygen can be written as 11H, 126C, and 168O, respectively.