The behavior of electrons determines chemical bonding and geometry
The number of electrons in an atom determines how it will combine with other atoms. Biologists are interested in how chemical changes take place in living cells. When considering atoms, they are concerned primarily with electrons because the behavior of electrons explains how chemical reactions occur. Chemical reactions alter the atomic compositions of substances and thus alter their properties. Reactions usually involve changes in the distribution of electrons between atoms.
The location of a given electron in an atom at any given time is impossible to determine. We can only describe a volume of space within the atom where the electron is likely to be. The region of space where the electron is found at least 90 percent of the time is the electron’s orbital. Orbitals have characteristic shapes and orientations, and a given orbital can be occupied by a maximum of two electrons. Thus any atom larger than helium (atomic number 2) must have electrons in two or more orbitals. As we move from lighter to heavier atoms in the periodic table, the orbitals are filled in a specific sequence, in a series of what are known as electron shells, or energy levels, around the nucleus.
First shell: The innermost electron shell consists of just one orbital. A hydrogen atom (1H) has one electron in its first shell; helium (2He) has two. Atoms of all other elements have two or more shells to accommodate orbitals for additional electrons.
Second shell: The second shell contains four orbitals and hence holds up to eight electrons.
27
Additional shells: Elements with more than ten electrons have three or more electron shells. The farther a shell is from the nucleus, the higher the energy level is for an electron occupying that shell.
Activity 2.1 Electron Orbitals
www.life11e.com/
The s orbitals fill with electrons first, and their electrons have the lowest energy level. Subsequent shells have different numbers of orbitals, but the outermost shells usually hold only eight electrons. In any atom, the outermost electron shell (the valence shell) determines how the atom combines with other atoms—
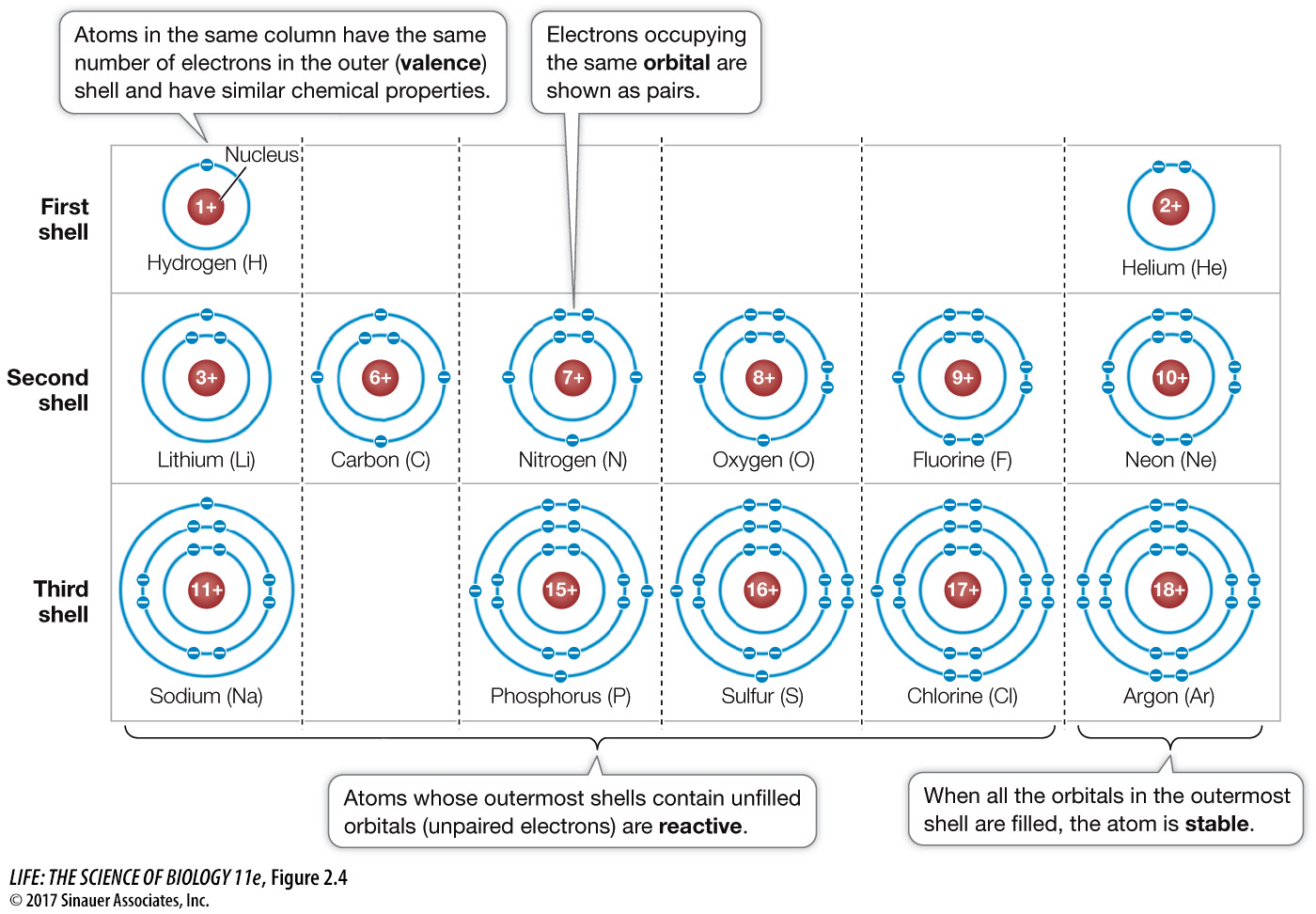
Atoms with unpaired electrons (i.e., partially filled orbitals) in their outermost electron shells are unstable and will undergo reactions in order to fill their outermost shells. Reactive atoms can attain stability either by sharing electrons with other atoms or by losing or gaining one or more electrons. In either case, the atoms involved are bonded together into stable associations called molecules. The tendency of atoms to form stable molecules so that they have eight electrons in their outermost shells is known as the octet rule. Many atoms in biologically important molecules—