The secondary structure of a protein requires hydrogen bonding
A protein’s secondary structure consists of regular, repeated spatial patterns in different regions of a polypeptide chain. There are two basic types of secondary structure, both determined by hydrogen bonding between the amino acids that make up the primary structure: the α helix and the β pleated sheet.
THE ALPHA HELIX The α (alpha) helix is a right-handed coil that turns in the same direction as a standard wood screw (see Figure 3.7B and Figure 3.8). The R groups extend outward from the peptide backbone of the helix. The coiling results from hydrogen bonds that form between the δ+ hydrogen of the N—H of one amino acid and the δ– oxygen of the C=O of another. When this pattern of hydrogen bonding is established repeatedly over a segment of the protein, it stabilizes the coil.
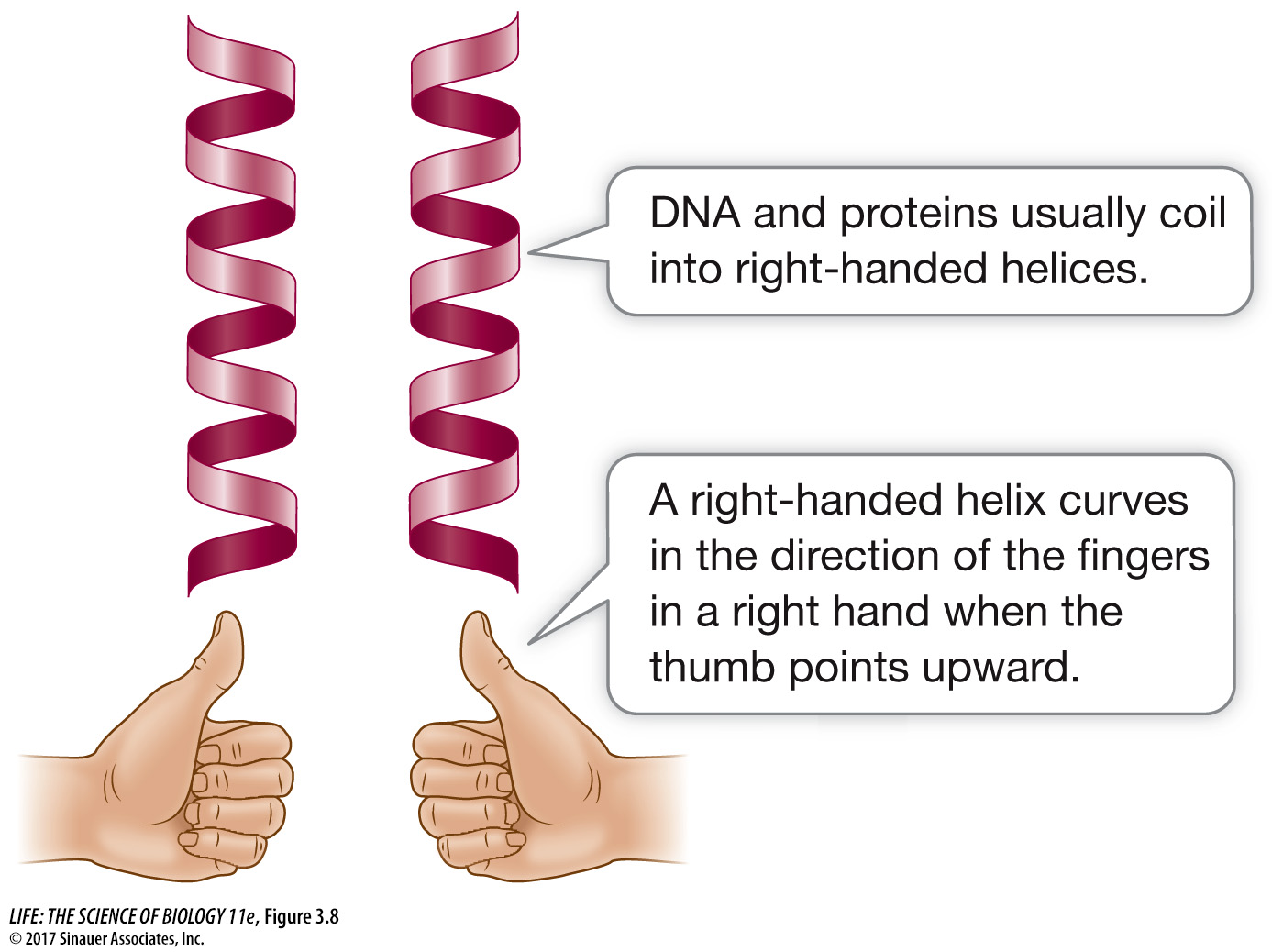
Figure 3.8 Left- and Right-Handed Helices A protein will often have one or more right-handed helices as part of its secondary structure.
THE BETA PLEATED SHEET A β (beta) pleated sheet is formed from two or more polypeptide chains that are almost completely extended and aligned. The sheet is stabilized by hydrogen bonds between the N—H groups on one chain and the C=O groups on the other (see Figure 3.7B). A β pleated sheet may form between separate polypeptide chains or between different regions of a single polypeptide chain that is bent back on itself. The ratcheted, stacked sheets in dragline spider silks (described at the beginning of the chapter and in Investigating Life: Making Spider Silk) are made up of β pleated sheets. Many proteins contain regions of both α helix and β pleated sheet in the same polypeptide chain.
