The tertiary structure of a protein is formed by bending and folding
In many proteins, the polypeptide chain is bent at specific sites and then folded back and forth, resulting in the tertiary structure of the protein (see Figure 3.7C). Although α helices and β pleated sheets contribute to the tertiary structure, usually only portions of the macromolecule have these secondary structures, and large regions consist of tertiary structure unique to a particular protein. For example, the proteins found in stretchy spider silks (see the opening story) have repeated amino acid sequences that cause the proteins to fold into spirals. Tertiary structure is a macromolecule’s definitive three-
The protein’s exposed outer surfaces present functional groups capable of interacting with other molecules in the cell. These molecules might be other macromolecules, including proteins, nucleic acids, carbohydrates, and lipid structures, or smaller chemical substances.
You saw that hydrogen bonding between the N—
Covalent disulfide bridges can form between specific cysteine side chains (see Figure 3.5), holding a folded polypeptide in place.
Hydrogen bonds between side chains also stabilize folds in proteins.
Hydrophobic side chains can aggregate together in the interior of the protein, away from water, folding the polypeptide in the process. Close interactions between the hydrophobic side chains are stabilized by van der Waals forces.
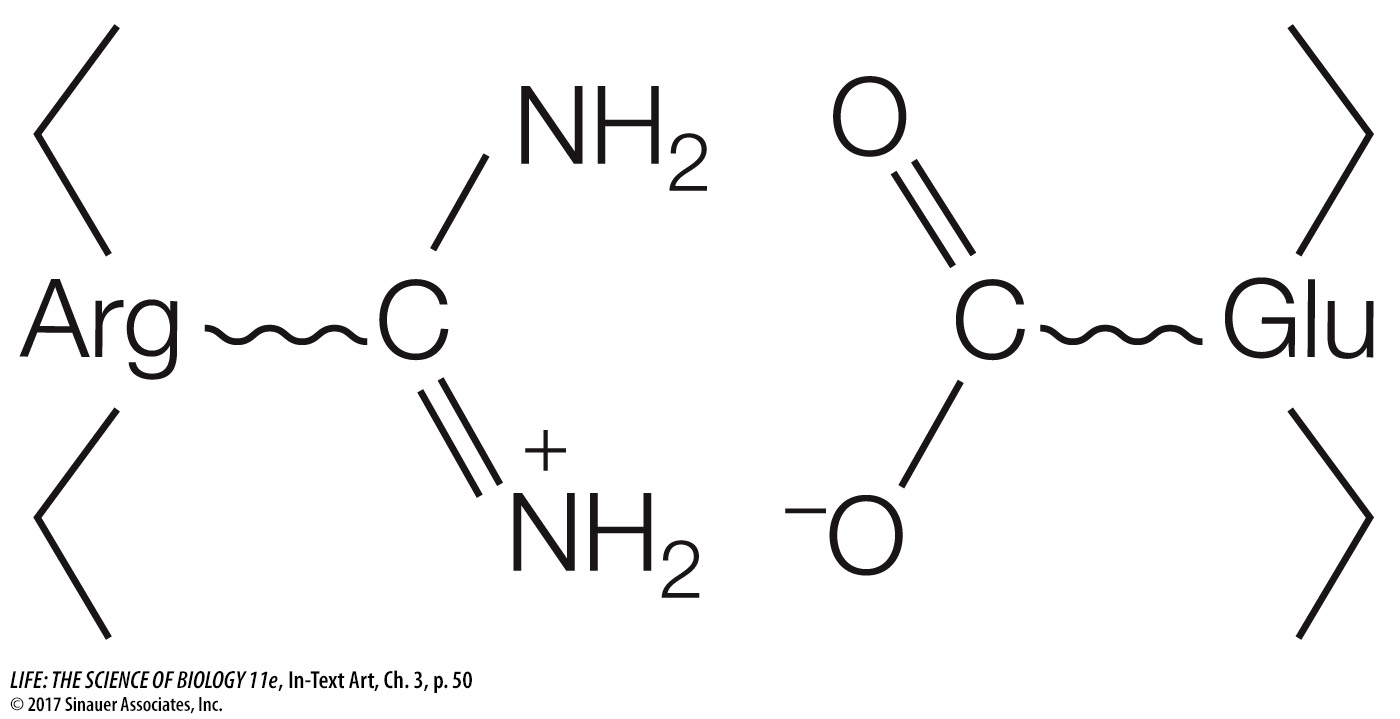
Ionic attractions can form between positively and negatively charged side chains, forming salt bridges between amino acids. Salt bridges can be near the surfaces of polypeptides or buried deep within a protein, away from water. These interactions occur between positively and negatively charged amino acids, for example glutamic acid (which has a negatively charged R group) and arginine (which is positively charged) (see Table 3.2):
50
A protein folds into its final shape in a way that maximizes all the interactions noted and minimizes inappropriate interactions, such as two positively charged residues (a term identifying monomers in a polymer) being near one another, or a hydrophobic residue being near water. A complete description of a protein’s tertiary structure would specify the location of every atom in the molecule in three-
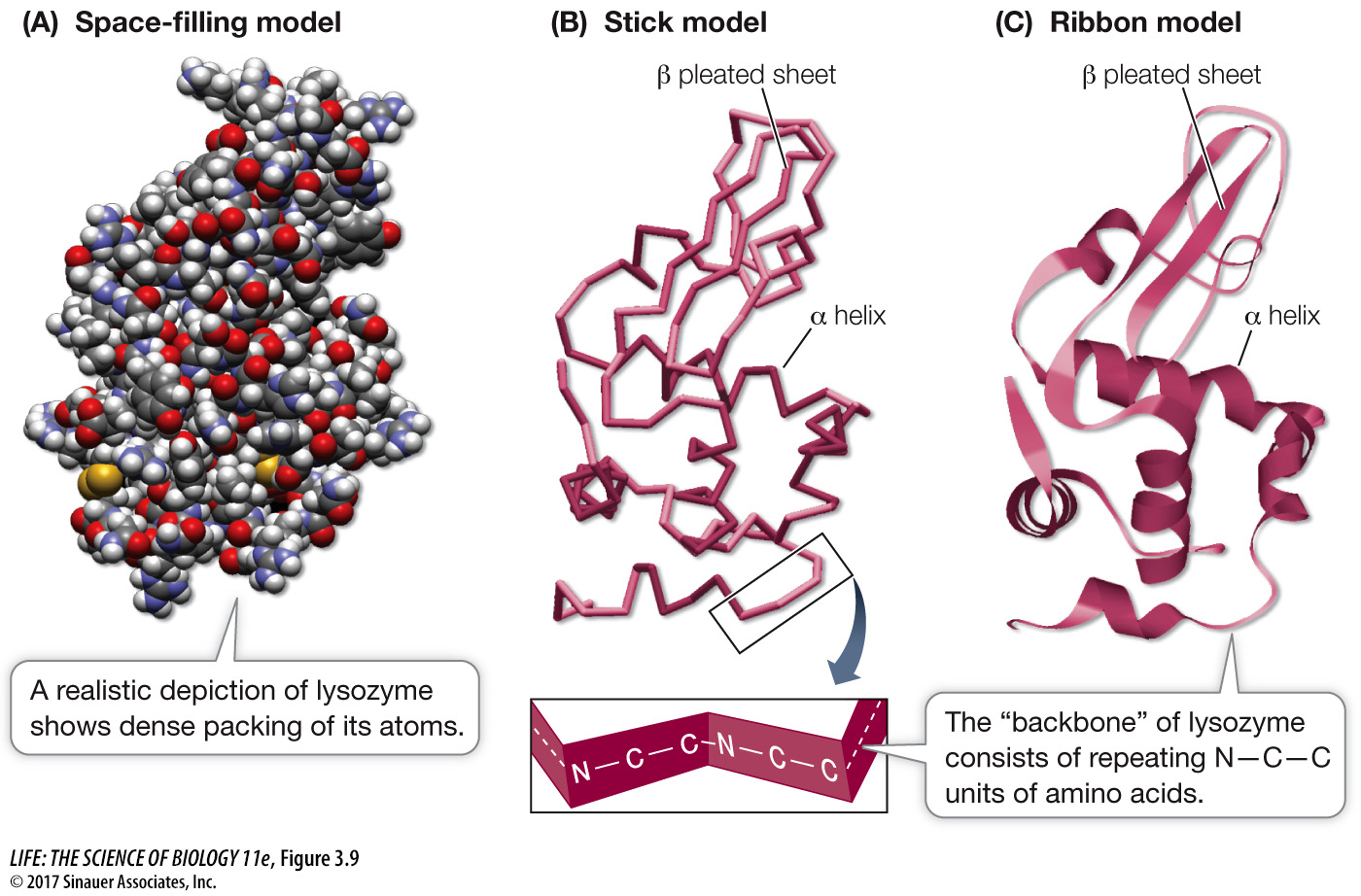
Question
Q: Can you identify regions of the protein that are hydrophilic? Hydrophobic?
Regions in lysozyme that face the outside (water) environment are hydrophilic, whereas those on the inside are generally hydrophobic.
Media Clip 3.1 Protein Structures in 3D
www.life11e.com/
Remember that both secondary and tertiary structure derive from primary structure. If a protein is heated slowly and moderately, the heat energy will disrupt only the weak interactions, causing the secondary and tertiary structure to break down. The protein is then said to be denatured. A comparison of native (untreated) and denatured proteins shows major differences:
Native proteins are compact; denatured proteins have a larger volume.
Native proteins exist in one, preferred shape; denatured proteins can take many shapes.
Native proteins have hydrogen bonds that stabilize the structure internally; denatured proteins have hydrogen bonds on the exterior, to water.
You can’t “unboil” an egg after it has been hard-
51
experiment
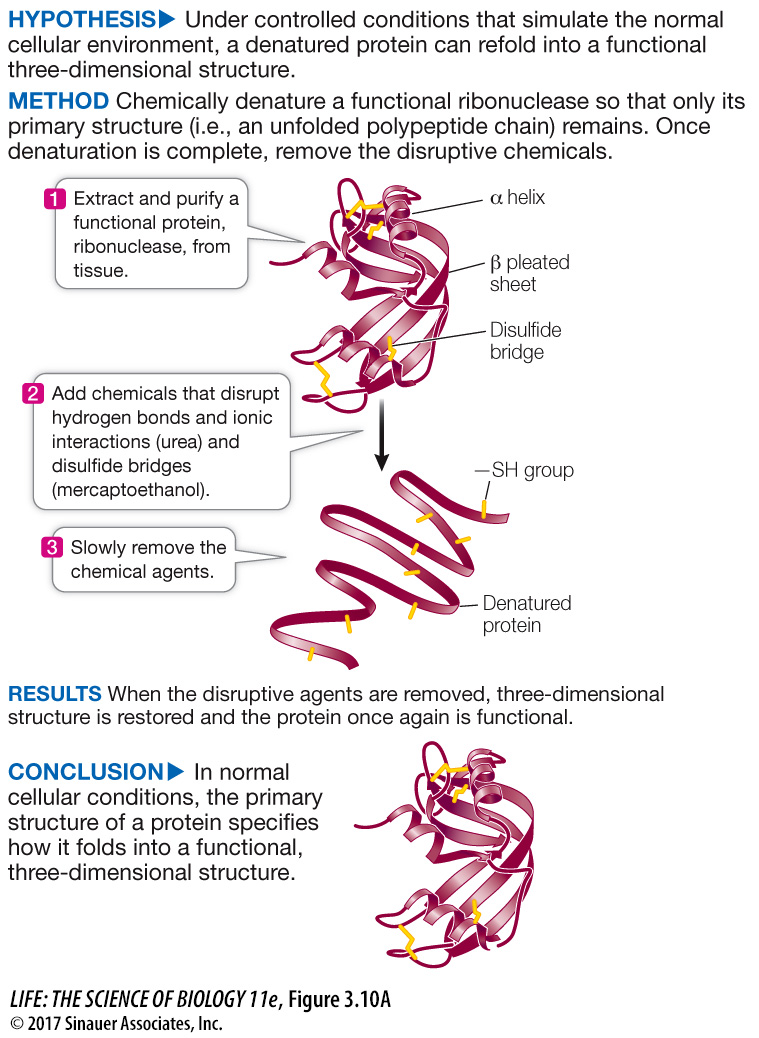
Figure 3.10A Primary Structure Specifies Tertiary Structure
Original Papers: Anfinsen, C. B., E. Haber, M. Sela and F. White, Jr. 1961. The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proceedings of the National Academy of Sciences USA 47: 1309–
White, Jr., F. 1961. Regeneration of native secondary and tertiary structures by air oxidation of reduced ribonuclease. Journal of Biological Chemistry 236: 1353–
Using the protein ribonuclease, Christian Anfinsen showed that proteins spontaneously fold into functionally correct three-
Figure 3.10B work with the data follows on next page.
52
work with the data
Figure 3.10B Primary Structure Specifies Tertiary Structure
Original Papers: Anfinsen, C. B. et al. 1961; White, Jr., F. 1961.
After the tertiary structures of proteins were shown to be highly specific, the question arose as to how the order of amino acids determined the three-
QUESTIONS
Question 1
Initially, the disulfide bonds (S—
Disulfide bonds began forming almost immediately after reoxidation began. Enzyme activity began appearing 100 minutes after reoxidation began. There are two reasons for the delay between the beginning of disulfide bond formation and the reappearance of enzyme activity. First, there are four disulfide bonds in the protein, all of which have to re-
At what time did disulfide bonds begin to form? At what time did enzyme activity begin to appear? Explain the difference between these times.
Question 2
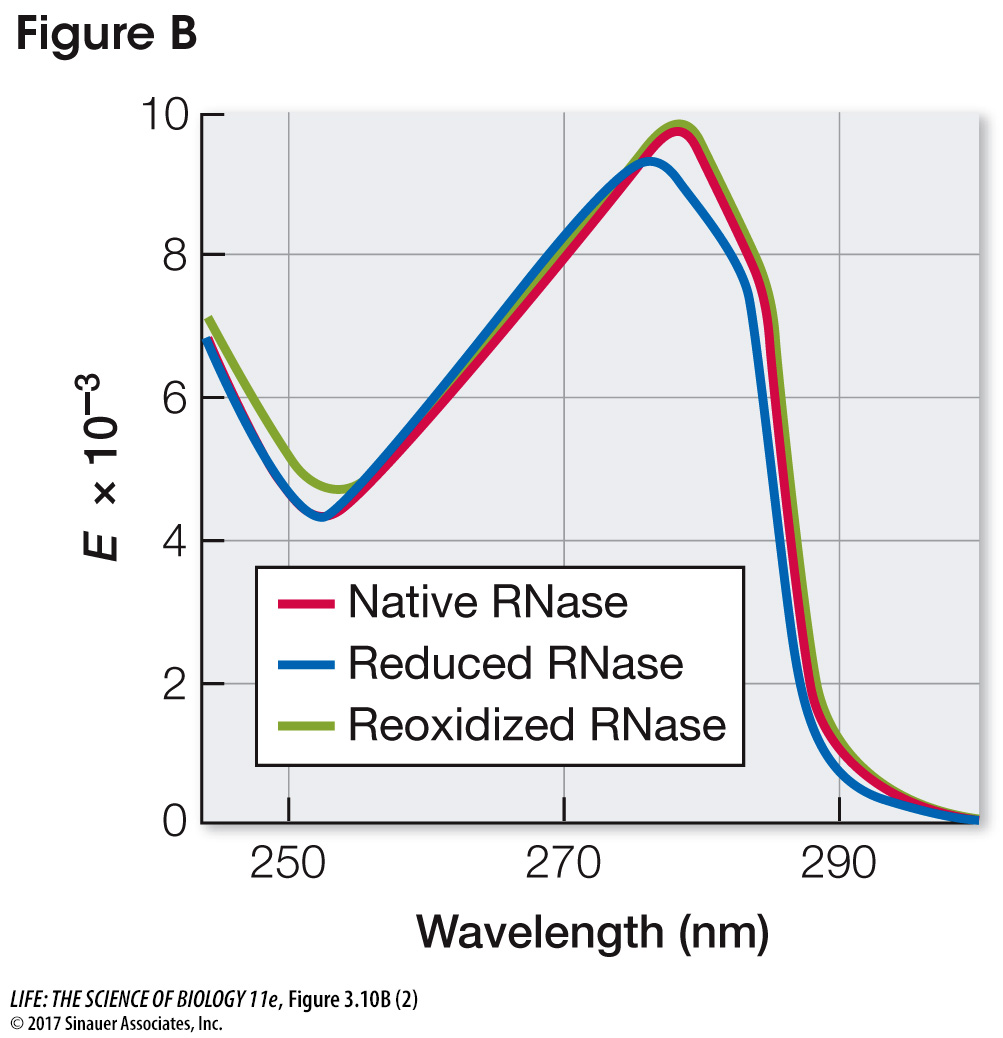
The three-
The absorption peak for the native protein was at about 278 nm; the peak for the reduced (denatured) protein was at about 275 nm. Reoxidation resulted in a return to the native spectrum. Under the denaturation conditions of these experiments, as long as the primary structure of RNase A is retained, the proper environmental conditions will result in a return to the native structure and a fully functional molecule.
Look carefully at the plots. What are the differences between the peak absorbances of native (untreated) and reduced (denatured) RNase A? What happened when reduced RNase A was reoxidized (renatured)? What can you conclude about the structure of RNase A from these experiments?

A similar work with the data exercise may be assigned in LaunchPad.