The quaternary structure of a protein consists of subunits
Many functional proteins contain two or more polypeptide chains, called subunits, each of them folded into its own unique tertiary structure. The protein’s quaternary structure results from the ways in which these subunits bind together and interact (see Figure 3.7D).
Hemoglobin has four polypeptide chains interacting to form quaternary structure (Figure 3.11). Hydrophobic interactions, van der Waals forces, hydrogen bonds, and ionic attractions all help hold the four subunits together to form a hemoglobin molecule. However, the weak nature of these forces permits small changes in the quaternary structure to aid the protein’s function—which is to carry oxygen in red blood cells. As hemoglobin binds one O2 molecule, the four subunits shift their relative positions slightly, changing the quaternary structure. Ionic attractions are broken, exposing buried side chains that enhance the binding of additional O2 molecules. The quaternary structure changes back when hemoglobin releases its O2 molecules to the cells of the body.
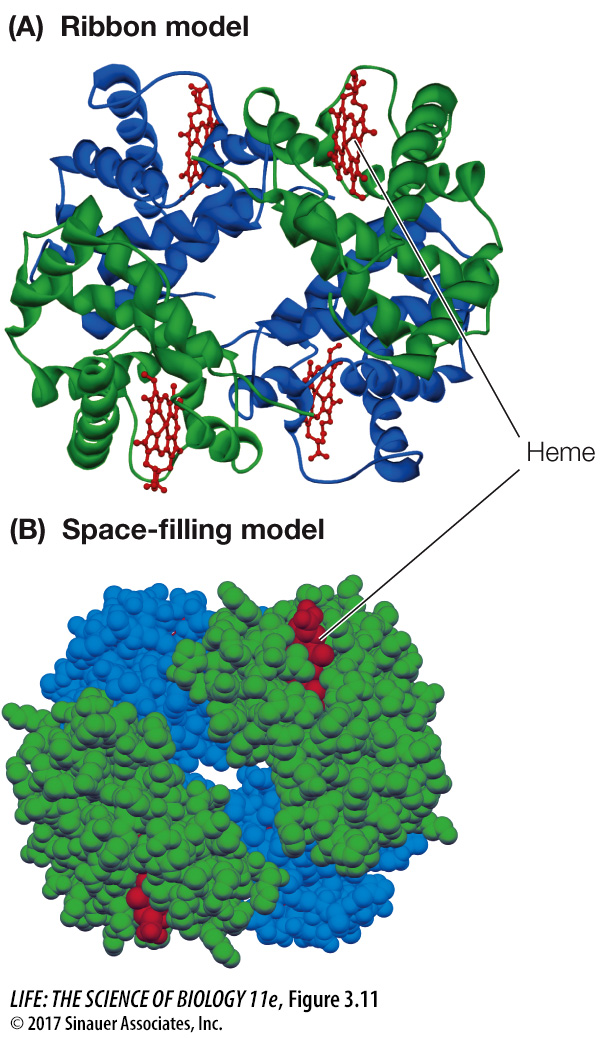
Figure 3.11 Quaternary Structure of a Protein Hemoglobin consists of four folded polypeptide subunits that assemble themselves into the quaternary structure represented by the ribbon model (A) and space-filling model (B). In both graphic representations, each type of subunit is a different color (α subunits are blue and β subunits are green). The heme groups (red) contain iron and are the oxygen-carrying sites.
