Shape and surface chemistry contribute to protein function
The shapes of proteins allow specific sites on their exposed surfaces to bind noncovalently to other molecules, which may be large or small. The binding is usually very specific because only certain compatible chemical groups will bind to one another. The specificity of protein binding depends on two general properties of the protein: its shape, and the chemistry of its exposed surface groups.
Shape. When a small molecule collides with and binds to a much larger protein, it is like a baseball being caught by a catcher’s mitt: the mitt has a shape that binds to the ball and fits around it. Just as a hockey puck or a ping-
pong ball does not fit a baseball catcher’s mitt, a given molecule will not bind to a protein unless there is a general “fit” between their three- dimensional shapes. Chemistry. The exposed R groups on the surface of a protein permit chemical interactions with other substances (Figure 3.12). Three types of interactions may be involved: ionic, hydrophobic, or hydrogen bonding. Many important functions of proteins involve interactions between surface R groups and other molecules.
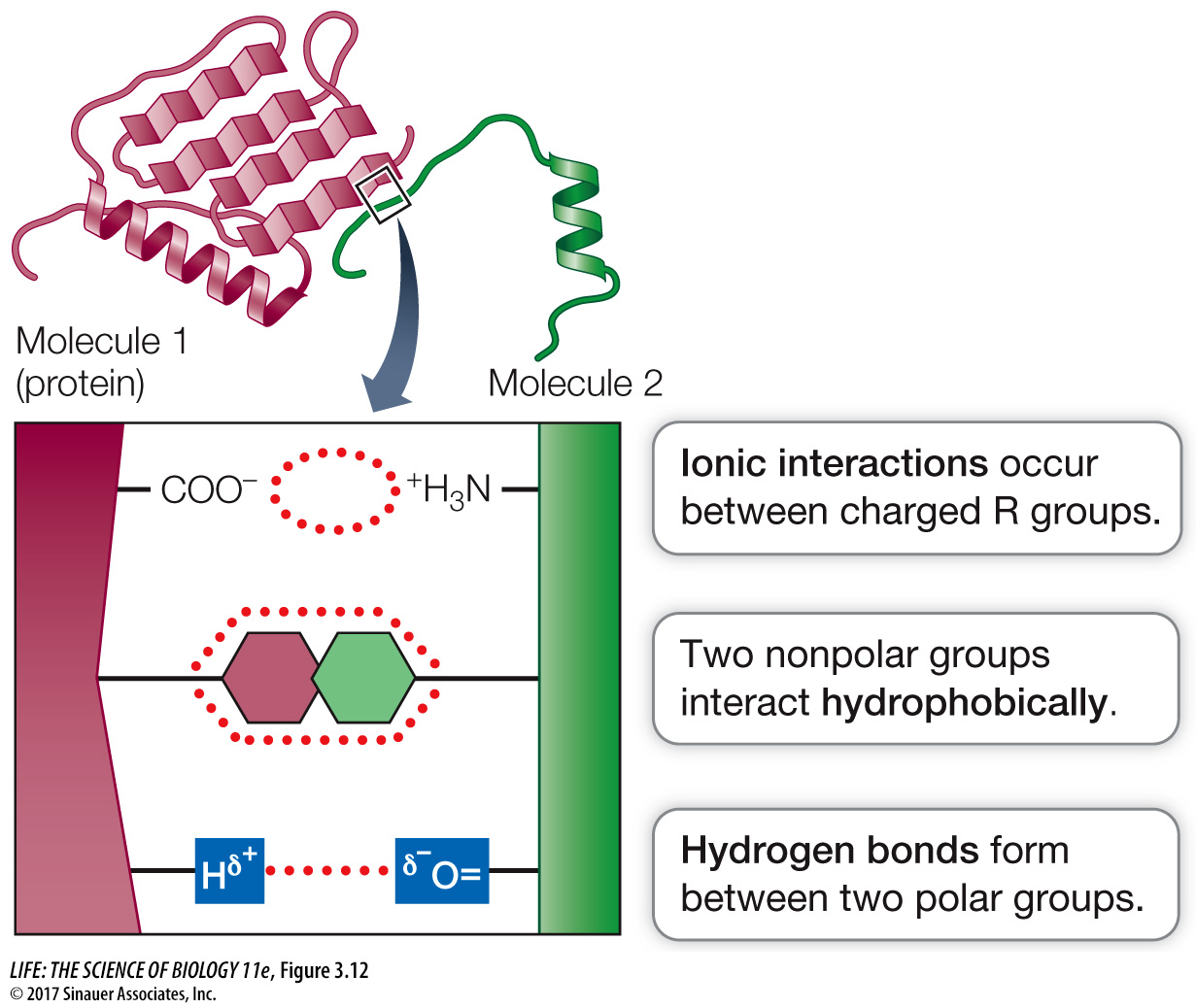 Figure 3.12 Noncovalent Interactions between Proteins and Other Molecules Noncovalent interactions (see p. 51) allow a protein (red) to bind tightly to another molecule (green) with specific properties. Noncovalent interactions also allow regions within the same protein to interact with one another.
Figure 3.12 Noncovalent Interactions between Proteins and Other Molecules Noncovalent interactions (see p. 51) allow a protein (red) to bind tightly to another molecule (green) with specific properties. Noncovalent interactions also allow regions within the same protein to interact with one another.Question
Q: Why are these interactions sensitive to heat? (Hint: see Table 2.1.)
Weak interactions shown within or between molecules require a relatively low amount of energy to break them apart.