Monosaccharides are simple sugars
All living cells contain the monosaccharide glucose; it is the “blood sugar” used to store and transport energy in humans. Cells use glucose as an energy source, breaking it down through a series of reactions that converts stored energy to more usable chemical energy and produce carbon dioxide; this is a cellular form of the combustion reaction you saw in Key Concept 2.3.
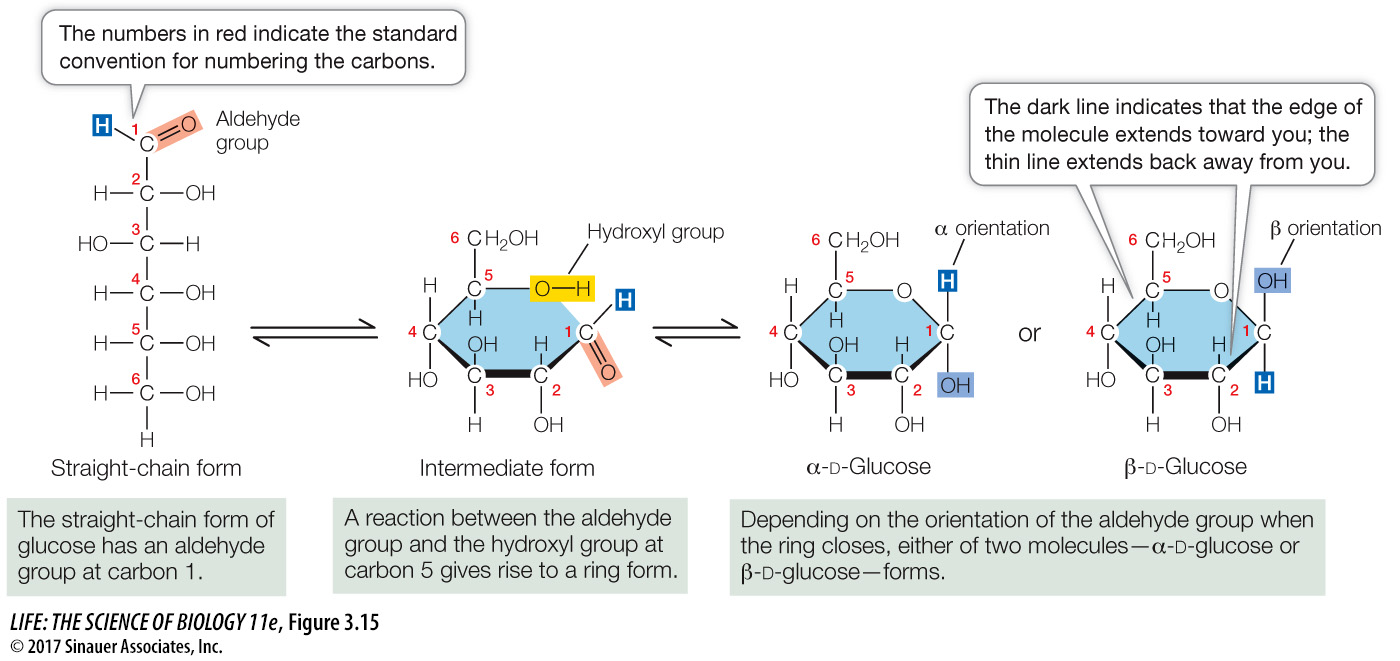
Glucose exists in straight chains and in ring forms. The ring forms predominate in virtually all biological circumstances because they are more stable in water. There are two versions of the glucose ring, called α- and β-glucose, which differ only in the orientation of the —H and —OH groups attached to carbon 1 (Figure 3.15). The α and β forms interconvert and exist in equilibrium when dissolved in water.
Activity 3.3 Forms of Glucose
www/
55
Different monosaccharides contain different numbers of carbons. Some monosaccharides are structural isomers, with the same kinds and numbers of atoms but in different arrangements (Figure 3.16). Such seemingly small structural changes can significantly alter their properties. Most of the monosaccharides in living systems belong to the D (right-
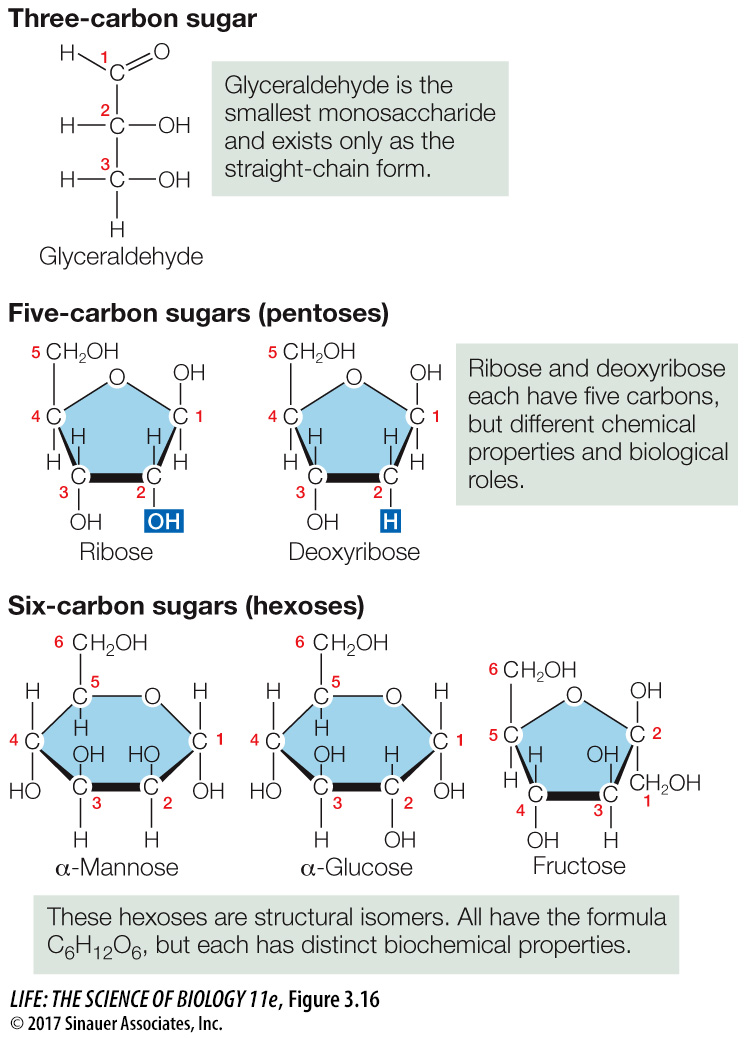
Pentoses (pente, “five”) are five-
carbon sugars. Two pentoses are of particular biological importance: the backbones of the nucleic acids RNA and DNA contain ribose and deoxyribose, respectively (see Key Concept 4.1). These two pentoses are not isomers of each other; rather, one oxygen atom is missing from carbon 2 in deoxyribose (de-, “absent”). The absence of this oxygen atom is an important distinction between RNA and DNA. The hexoses (hex, “six”) shown in Figures 3.15 and 3.16 are a group of structural isomers with the formula C6H12O6. Common hexoses are glucose, fructose (so named because it was first found in fruits), mannose, and galactose.