Glycosidic linkages bond monosaccharides
The disaccharides, oligosaccharides, and polysaccharides are all constructed from monosaccharides that are covalently bonded together by condensation reactions that form glycosidic linkages (Figure 3.17). A single glycosidic linkage between two monosaccharides forms a disaccharide. For example, sucrose—
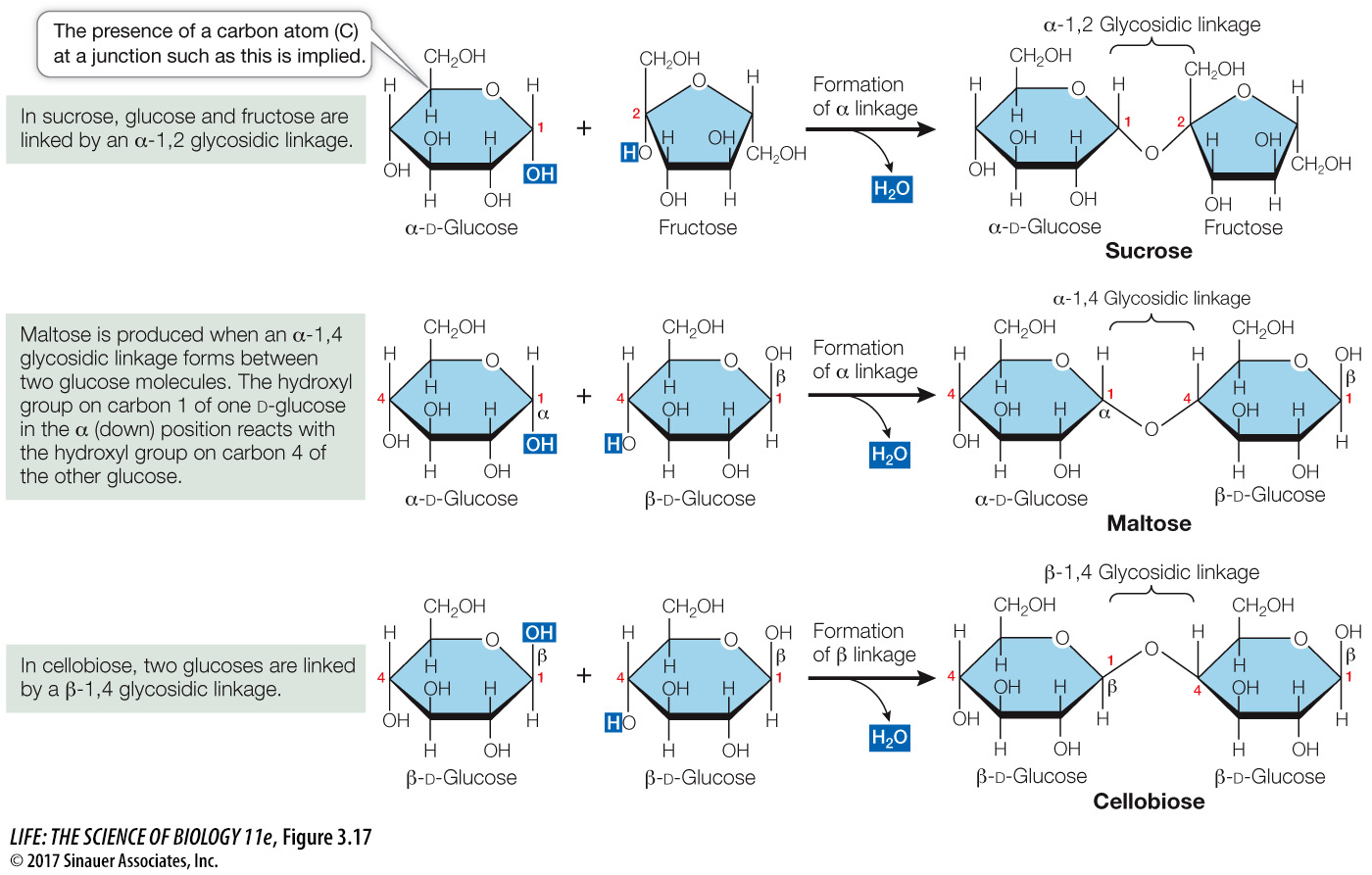
The disaccharides maltose and cellobiose are made from two glucose molecules (see Figure 3.17). Maltose and cellobiose are structural isomers, both having the formula C12H22O11. However, they have different chemical properties and are recognized by different *enzymes in biological tissues. For example, maltose can be hydrolyzed into its monosaccharides in the human body, whereas cellobiose cannot.
*connect the concepts Enzymes are an important class of proteins that may change shape when they come into contact with a reactant in a biochemical reaction. Each enzyme is specific for the reactant it binds. See Key Concept 8.4.
56
Oligosaccharides contain several monosaccharides bound by glycosidic linkages at various sites. Many oligosaccharides have additional functional groups, which give them special properties. Oligosaccharides are often covalently bonded to proteins and lipids on the outer cell surface, where they serve as recognition signals. The different human blood groups (e.g., the ABO blood types) get their specificities from oligosaccharide chains.