Polysaccharides store energy and provide structural materials
Polysaccharides are large (sometimes gigantic) polymers of monosaccharides connected by glycosidic linkages (Figure 3.18). In contrast to polypeptides, polysaccharides are not necessarily linear chains of monomers. Each monomer unit has several sites that are capable of forming glycosidic linkages, and thus branched molecules are possible.
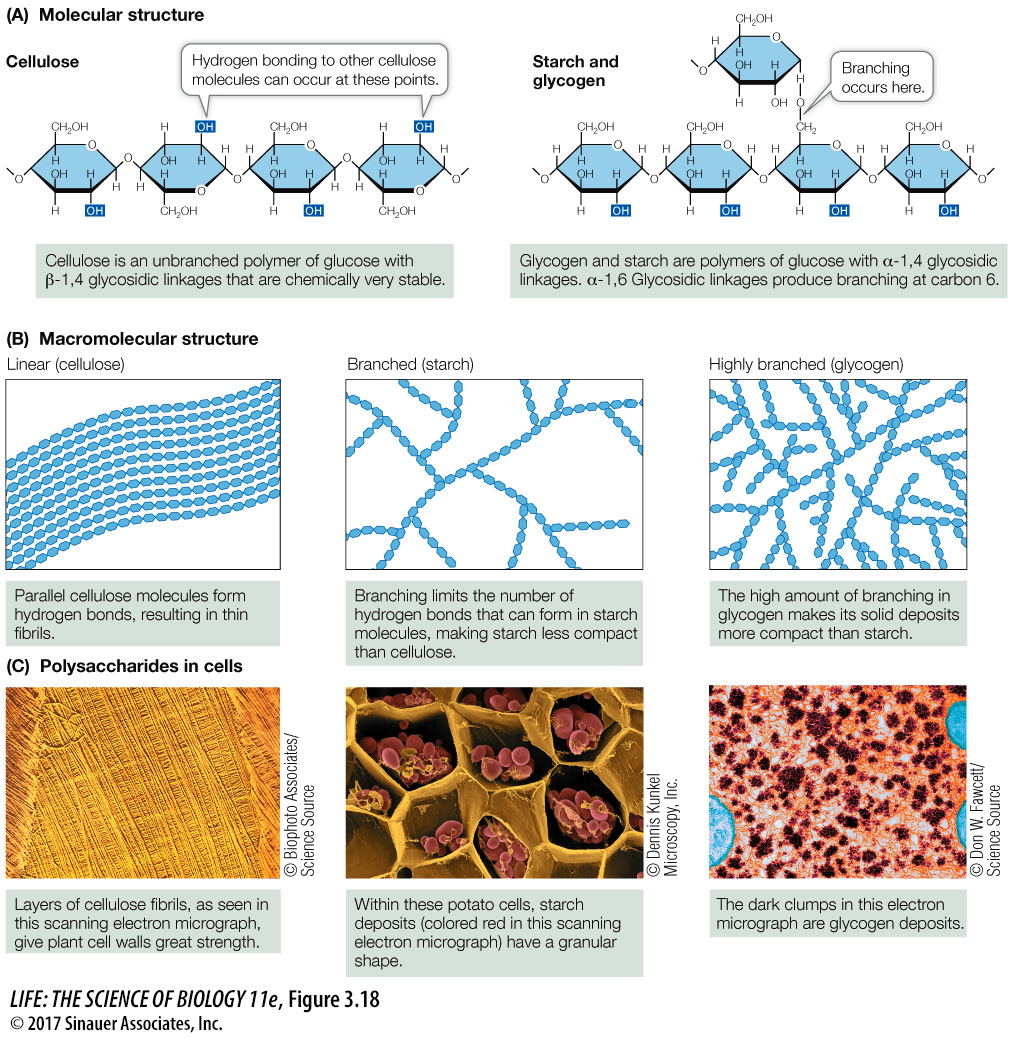
STARCH Starches comprise a family of large molecules with similar structures. While all starches are polysaccharides of glucose with α-glycosidic linkages (α–1,4 and α–1,6 glycosidic bonds; see Figure 3.18A), the different starches can be distinguished by the amount of branching that occurs at carbons 1 and 6 (see Figure 3.18B). Starch is the principal energy storage compound of plants. Some plant starches, such as amylose, are unbranched; others are moderately branched (for example, amylopectin). Starch readily binds water; if you’re a cook you know this. However, when water is removed, hydrogen bonds form between the unbranched polysaccharide chains, which then aggregate. Large starch aggregates called starch grains can be observed in the storage tissues of plant seeds (see Figure 3.18C). These aggregates are broken up when starch is heated, breaking the hydrogen bonds. The starch becomes less solid and crystalline and water is absorbed, making the starch even more amorphous. This is what happens in baking with wheat flour, and is what gives bread its texture. Next time you eat some bread, think of hydrogen bonds!
GLYCOGEN Glycogen is a water-
But if it is glucose that is needed for fuel, why store it in the form of glycogen? The reason is that 1,000 glucose molecules would exert 1,000 times the osmotic pressure of a single glycogen molecule, causing water to enter cells where glucose is stored (see Key Concept 6.3). If it were not for polysaccharides, many organisms would expend a lot of energy expelling excess water from their cells.
57
CELLULOSE As the predominant component of plant cell walls, cellulose is by far the most abundant organic compound on Earth. Like starch and glycogen, cellulose is a polysaccharide of glucose, but its individual monosaccharides are connected by β- rather than by α-glycosidic linkages. Starch is easily degraded by the actions of chemicals or enzymes. Cellulose, however, is chemically more stable because of its β-glycosidic linkages. Thus whereas starch is easily broken down to supply glucose for energy-