Phospholipids form biological membranes
We have mentioned the hydrophobic nature of the many C—C and C—H bonds in fatty acids. But what about the carboxyl functional group at the end of the molecule? When it ionizes and forms COO–, it is strongly hydrophilic. So a fatty acid is a molecule with a hydrophilic end and a long hydrophobic “tail.” It has two opposing chemical properties—part hydrophobic and part hydrophilic; the technical term for this is amphipathic.
Like triglycerides, phospholipids contain fatty acids bound to glycerol by ester linkages. In phospholipids, however, any one of several phosphate-containing compounds replaces the first or third fatty acid, giving phospholipids amphipathic properties (Figure 3.22A). The phosphate functional group has a negative electric charge, so this portion of the molecule is hydrophilic, attracting polar water molecules. But the two fatty acids are hydrophobic, so they tend to avoid water and aggregate together or with other hydrophobic substances.
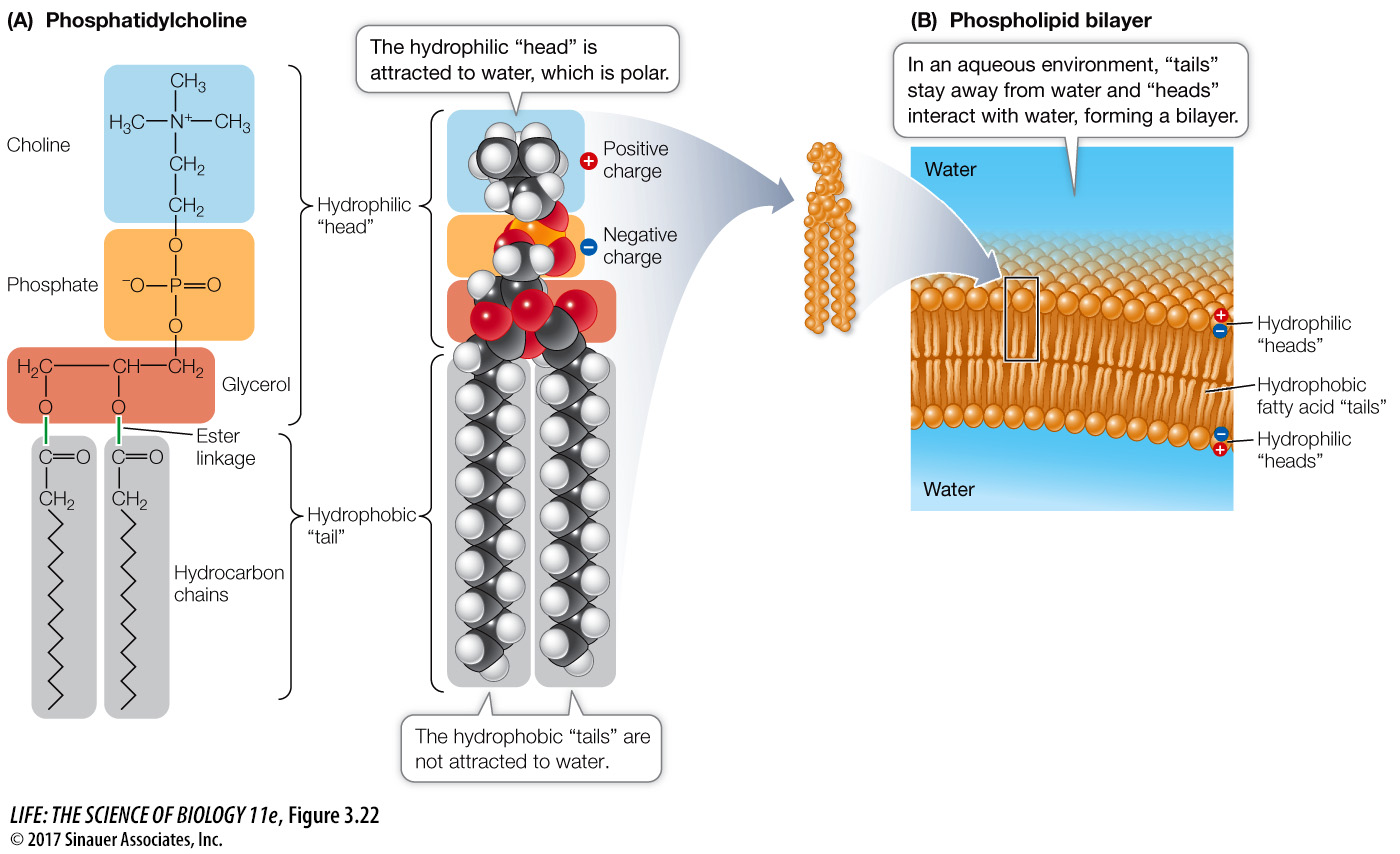
Figure 3.22 Phospholipids (A) Phosphatidylcholine (lecithin) demonstrates the structure of a phospholipid molecule. In other phospholipids, the amino acid serine, the sugar alcohol inositol, or other compounds replace choline. (B) In an aqueous environment, hydrophobic interactions bring the “tails” of phospholipids together in the interior of a bilayer. The hydrophilic “heads” face outward on both sides of the bilayer, where they interact with the surrounding water molecules.
In an aqueous environment, phospholipids line up in such a way that the nonpolar, hydrophobic “tails” pack tightly together and the phosphate-containing “heads” face outward, where they interact with water. The phospholipids can form a bilayer: a sheet two molecules thick, with water excluded from the core (Figure 3.22B). Biological membranes have this kind of phospholipid bilayer structure, and we will devote Chapter 6 to their biological functions.
