Chemical groupings determine the structures of macromolecules
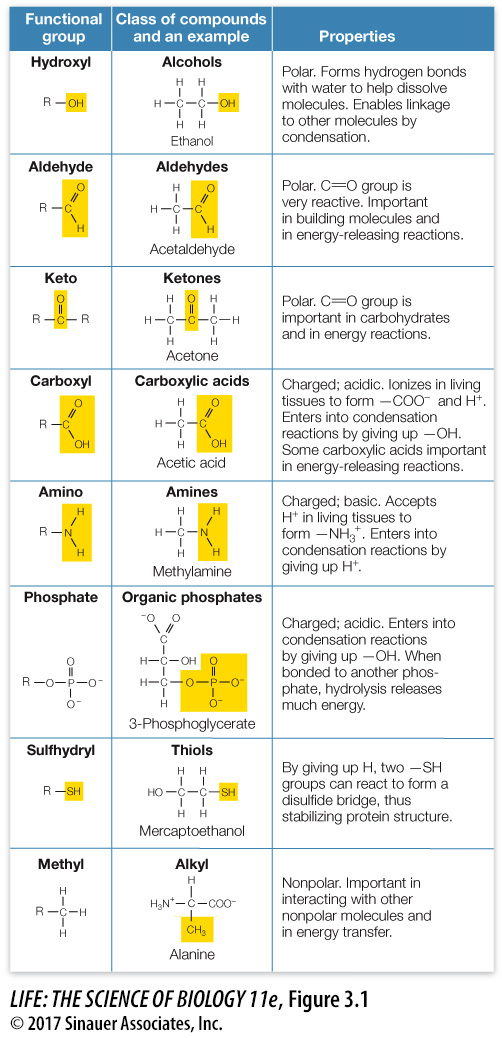
Certain small groups of atoms, called functional groups, occur frequently in biological molecules (Figure 3.1). Each functional group has specific chemical properties, and when it is attached to a larger molecule, it confers those properties on the larger molecule. One of these properties is polarity. Looking at the structures in Figure 3.1, can you determine which functional groups are the most polar? (Hint: look for C—
43
Activity 3.1 Functional Groups
www.life11e.com/
Animation 3.1 Proteins, Carbohydrates, and Lipids
www.life11e.com/
Macromolecules have many different functional groups. A single large protein may contain nonpolar, polar, and charged functional groups, each of which gives different specific properties to local sites on the macromolecule. As we will see, sometimes these different groups interact within the same macromolecule. They help determine the shape of the macromolecule as well as how it interacts with other macromolecules and with smaller molecules.
Using the same atoms, molecules can differ from one another because their functional groups can be arranged differently. Isomers are molecules that have the same (“iso”) chemical formula—
Structural isomers differ in how their atoms are joined together. Consider two simple molecules, each composed of four carbon and ten hydrogen atoms bonded covalently, both with the formula C4H10. These atoms can be linked in two different ways, resulting in different molecules (Figure 3.2A).
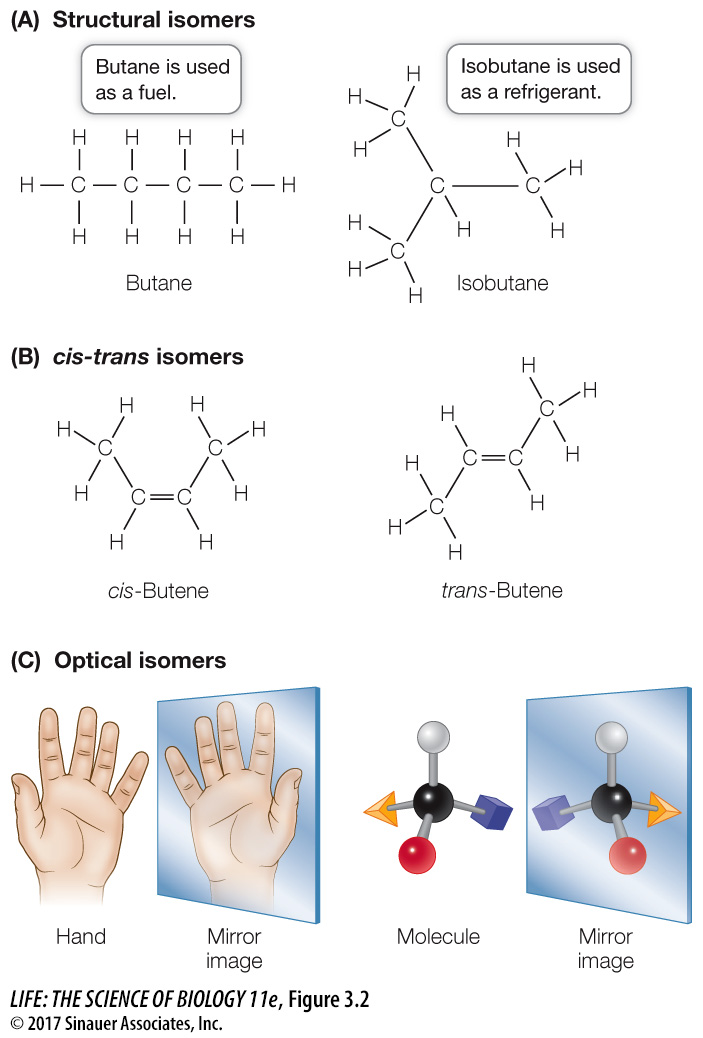 Figure 3.2 Isomers Isomers have the same chemical formula, but the atoms are arranged differently. Pairs of isomers often have different chemical properties and functions.
Figure 3.2 Isomers Isomers have the same chemical formula, but the atoms are arranged differently. Pairs of isomers often have different chemical properties and functions.cis-
trans isomers typically involve a double bond between two carbon atoms, where the carbons share two pairs of electrons. When the remaining two bonds of each of these carbons are to two different atoms or groups of atoms (e.g., a hydrogen and a methyl group; Figure 3.2B), these can be oriented on the same side or different sides of the double- bonded molecule. If the different atoms or groups of atoms are on the same side, the double bond is called cis; if they are on opposite sides, the bond is trans. These molecules can have very different properties. Optical isomers occur when a carbon atom has four different atoms or groups of atoms attached to it. This pattern allows for two different ways of making the attachments, each the mirror image of the other (Figure 3.2C). Such a carbon atom is called an asymmetric carbon, and the two resulting molecules are optical isomers of one another. You can envision your right and left hands as optical isomers. Just as a glove is specific for a particular hand, some biochemical molecules that can interact with one optical isomer of a carbon compound are unable to “fit” the other.