Most macromolecules are formed by condensation and broken down by hydrolysis
Polymers are formed from monomers by a series of condensation reactions (in this case called dehydration reactions; both terms refer to the loss of water). Condensation reactions result in the formation of covalent bonds between monomers. A molecule of water is released with each covalent bond formed (Figure 3.4A). The condensation reactions that produce the different kinds of polymers differ in detail, but in all cases polymers form only if water molecules are removed and energy is added to the system. In living systems, specific energy-
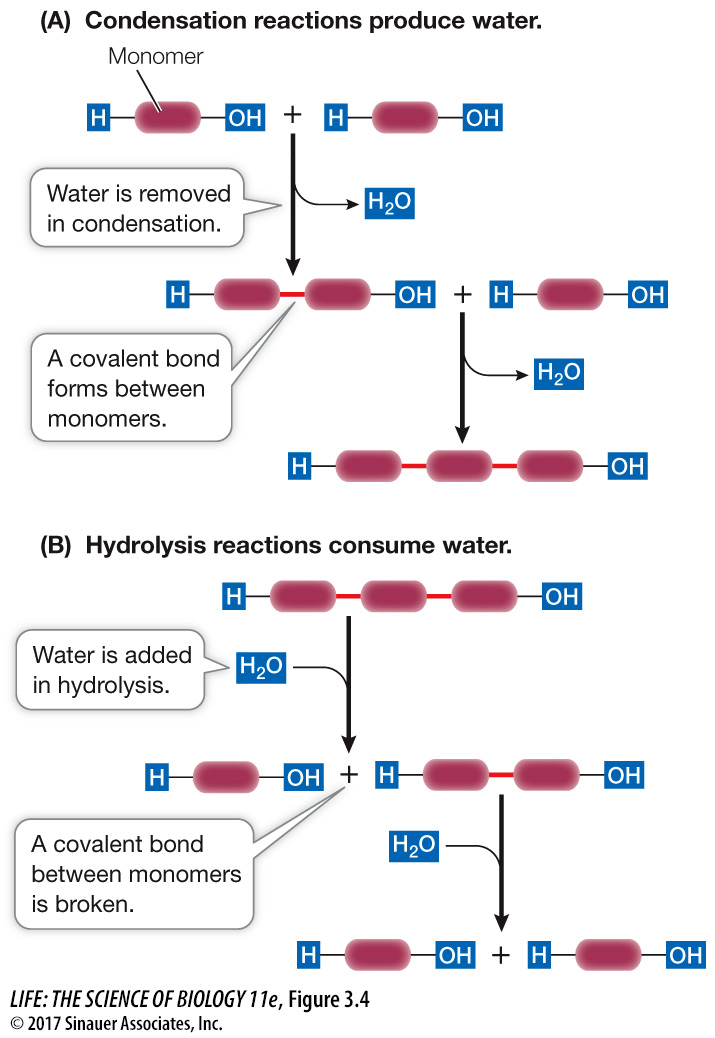
The reverse of a condensation reaction is a hydrolysis reaction (hydro, “water,” + lysis, “break”). Hydrolysis reactions result in the breakdown of polymers into their component monomers. Water reacts with the covalent bonds that link the polymer together. For each covalent bond that is broken, a water molecule splits into two ions (H+ and OH–), each of which becomes part of one of the products (Figure 3.4B).
The four types of macromolecules can be seen as the building blocks of life. We will cover the unique properties of the nucleic acids in Chapter 4. The remainder of this chapter will describe the structures and functions of the proteins, carbohydrates, and lipids.