Prebiotic synthesis experiments model early Earth
One theory for the origin of life on Earth, chemical evolution, holds that conditions on primitive Earth led to the formation of simple molecules such as monomers (see Key Concept 3.1), and that these molecules led to the formation of life forms. Scientists have sought to reconstruct those primitive conditions, both physically (by varying temperature) and chemically (by re-
HOT CHEMISTRY In oxygenated water, some trace metals such as molybdenum and rhenium are soluble, and their presence in sediments under oceans and lakes is directly proportional to the amount of oxygen gas (O2) that was present in and above the water at the times the rocks were formed. Measurements of dated sedimentary cores indicate that none of these rare metals was present prior to 2.5 billion years ago. This and other lines of evidence suggest that there was little O2 in Earth’s early atmosphere. Oxygen gas is thought to have accumulated about 2.5 billion years ago as the by-
In the 1950s Stanley Miller and Harold Urey at the University of Chicago set up an experimental “atmosphere” containing the gases they thought were present in Earth’s early atmosphere: hydrogen gas, ammonia, methane gas, and water vapor. They passed an electrical spark through these gases to simulate lightning, a source of energy to drive chemical reactions. Then they cooled the system so the gases would condense and collect in a watery solution, or “ocean” (Figure 4.7). After a week of continuous operation, the system contained numerous organic molecules, including a variety of amino acids—
72
experiment
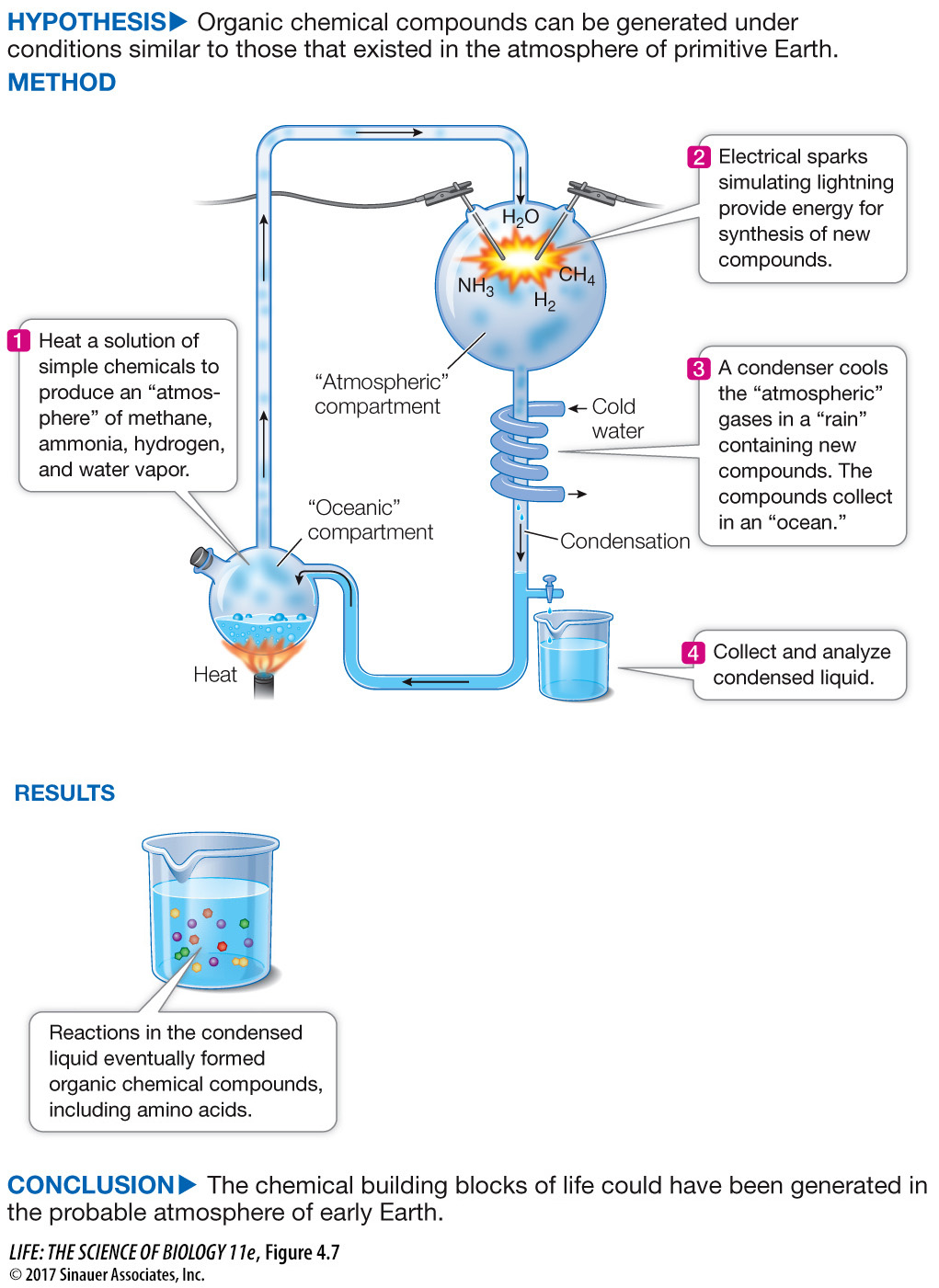
Figure 4.7 Could Biological Molecules Have Been Formed from Chemicals Present in Earth’s Early Atmosphere?
Original Papers: Miller, S. L. 1953. A production of amino acids under possible primitive earth conditions. Science 117: 528–
Miller, S. L. and H. C. Urey. 1959. Organic compound synthesis on the primitive earth. Science 130: 245–
With an increased understanding of the atmospheric conditions that existed on primitive Earth, the researchers devised an experiment to see if these conditions could lead to the formation of organic molecules.
Animation 4.3 Synthesis of Prebiotic Molecules
www.life11e.com/
COLD CHEMISTRY Stanley Miller also performed a long-
73
The results of these experiments were profoundly important in giving weight to speculations about the chemical origin of life on Earth and elsewhere in the universe. Decades of experimental work and critical evaluation followed Miller and Urey’s original experiments. In science, an experiment and its results must be repeatable and be reinterpreted and refined as more knowledge accumulates. For example, ideas about Earth’s original atmosphere have changed. There is abundant evidence indicating that major volcanic eruptions occurred 4 billion years ago; these would have released carbon dioxide (CO2), nitrogen (N2), hydrogen sulfide (H2S), and sulfur dioxide (SO2) into the atmosphere. Experiments using these gases in addition to the ones in the original Miller–
All five bases that are present in DNA and RNA (i.e., A, T, C, G, and U)
All of the 20 amino acids used in protein synthesis
Many three-
to six- carbon sugars Certain fatty acids
Vitamin B6 (pantothenic acid, a component of coenzyme A)
Nicotinamide (part of NAD, which is involved in energy metabolism)
Carboxylic acids such as succinic and lactic acids (also involved in energy metabolism)