Life may have come from outside Earth
In 1969 a remarkable event led to the discovery that a meteorite from space carried molecules that were characteristic of life on Earth. On September 28 of that year, fragments of a meteorite fell around the town of Murchison, Australia. Using gloves to avoid Earth-

Media Clip 4.1 DNA Building Blocks from Space
www.life11e.com/
Were these molecules truly brought from space as part of the meteorite, or did they get there after the rock landed on Earth? There are a number of reasons to believe the molecules were not Earthly contaminants:
The scientists took great care to avoid contamination. They used gloves and sterile instruments, took pieces from below the rock’s surface, and did their work very soon after the meteorite landed (they hoped before organisms from Earth could contaminate the samples).
Amino acids in most living organisms on Earth are L-amino acids; that is, the amino acids of living things are found in only one of the two possible optical isomeric forms (see Figure 3.2). The amino acids in the meteorite, however, were a mixture of L- and D-isomers, with a slight majority of the L form. Thus the amino acids in the meteorite were not likely to have come from a living organism on Earth.
In the story that opened Chapter 2, we described how the ratio of isotopes in a living organism reflects the ratio of the same isotopes in the environment where the organism lives. The isotope ratios for carbon and hydrogen in the sugars from the meteorite were different from the ratios of those elements found on Earth.
More than 90 meteorites from Mars have been recovered on Earth. Many show signs of water—
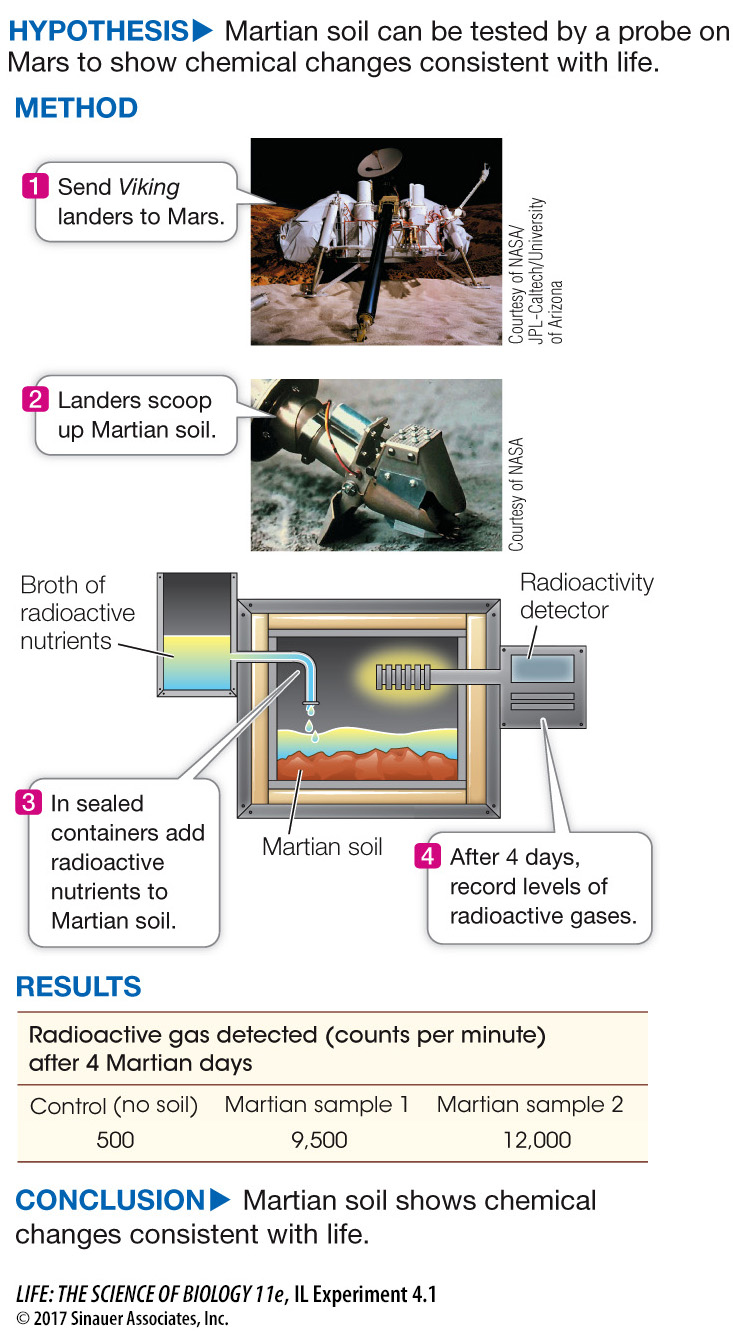
Life does not have to reach Earth from space to prove it exists elsewhere. There is an ongoing search for life in celestial bodies. For example, as mentioned in the opening story of this chapter, both orbiting satellites and vehicles probing the landscape of Mars search for conditions suitable for present or past life on Mars. Perhaps the most dramatic attempt to discover life on Mars came in 1976, when NASA celebrated the 200th anniversary of the political independence of the United States by landing two stationary probes, or landers, called Viking 1 and Viking 2 on the planet. Investigating Life: Can We Find Evidence of Life on Mars? describes how scientists on Earth directed onboard instruments to evaluate soil on Mars for signs of life.
74
investigating life
Can We Find Evidence of Life on Mars?
experiment
Original Papers: Levin, G. V. and O. Straat. 1976. Viking labeled release biology experiment: Interim results. Science 194: 1322–
Ponnamperuma, C., A. Shimoyama, M. Yamada, T. Hobo and R. Pal. 1977. Possible surface reactions on Mars: Implications for Viking biology results. Science 197: 455–
The Viking landers on Mars scooped up some of the soil and tested it for chemical interconversions consistent with life. Gilbert Levin and colleagues designed experiments to show remotely, at a distance of millions of miles, that there could be life on Mars at the present time.
work with the data
While it’s challenging to design experiments in the lab here on Earth, imagine the challenge of designing experiments done remotely on Mars! A team of biologists and chemists led by Levin and Straat sent instruments to Mars to test for the presence of life by measuring the emission of gases after nutrients were supplied to soils possibly containing Martian organisms. A scoop was used to obtain a 0.2 mL sample of soil which was then placed in a sealed container. The mixture was warmed to about 18oC (much higher than the surrounding Martian environment). Radioactive nutrients (formate, glycolate, glycine, alanine, and lactate) with their carbon atoms labeled in 14C were added. At intervals after nutrient injection, a radioactive detector was employed to test for the release of 14C radioactive gases.
QUESTIONS
Question 1
Table A shows data from two of the experiments. Plot the data as 14C radioactive gas versus Martian days after nutrient addition. What can you conclude? Laboratory experiments prior to the two Viking departures showed that the total radioactivity that could be released if all of the nutrients were completely converted to gases was 257,000 counts per minute (cpm). Calculate the fraction of this that appeared in the Martian sample and comment on the result.
Plot the data. In both experiments with unheated soil, there was a modest time-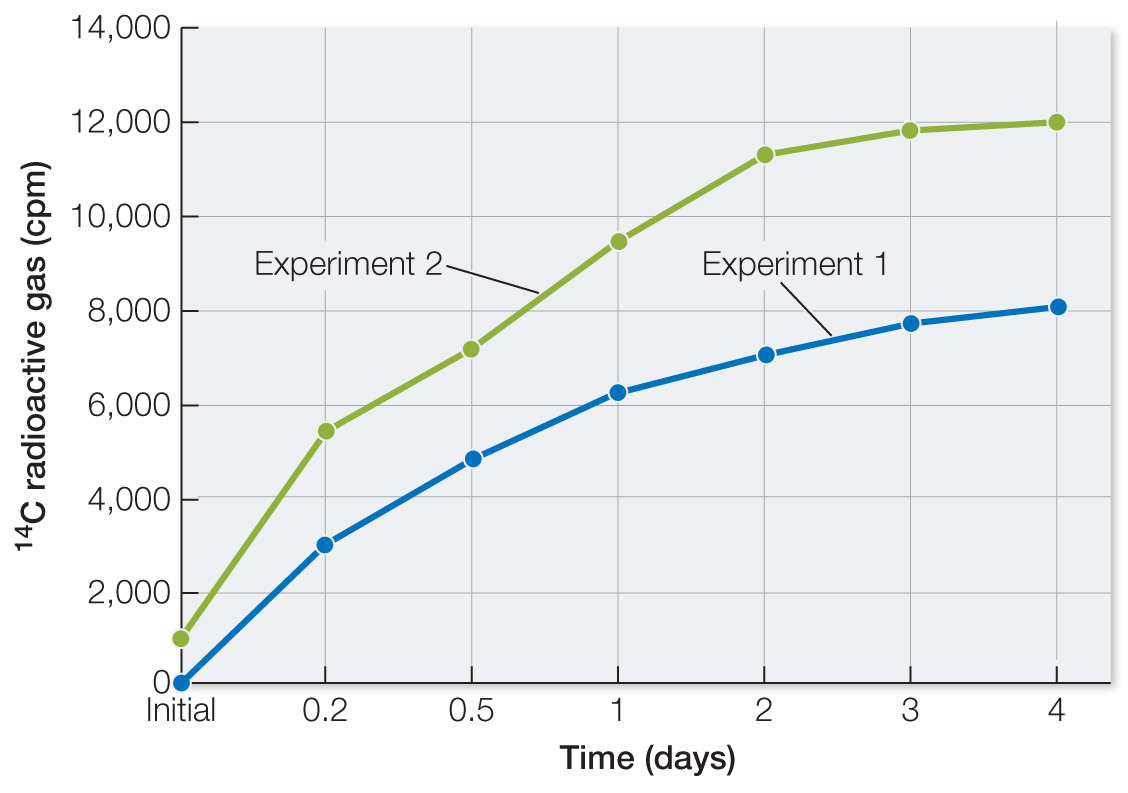
Question 2
Table A shows data from Martian soil that had been heated for 3 h at 160°C before nutrient addition. Plot these data on the same graph you made for Question 1. What types of molecules are destroyed by heat, and how does this occur? How do these data affect your conclusions from question 1?
The graph of the data shows no increase in 14C gases. Heat destroys hydrogen bonds in proteins and nucleic acids. Again, these data are consistent with living organisms having produced 14C gases. 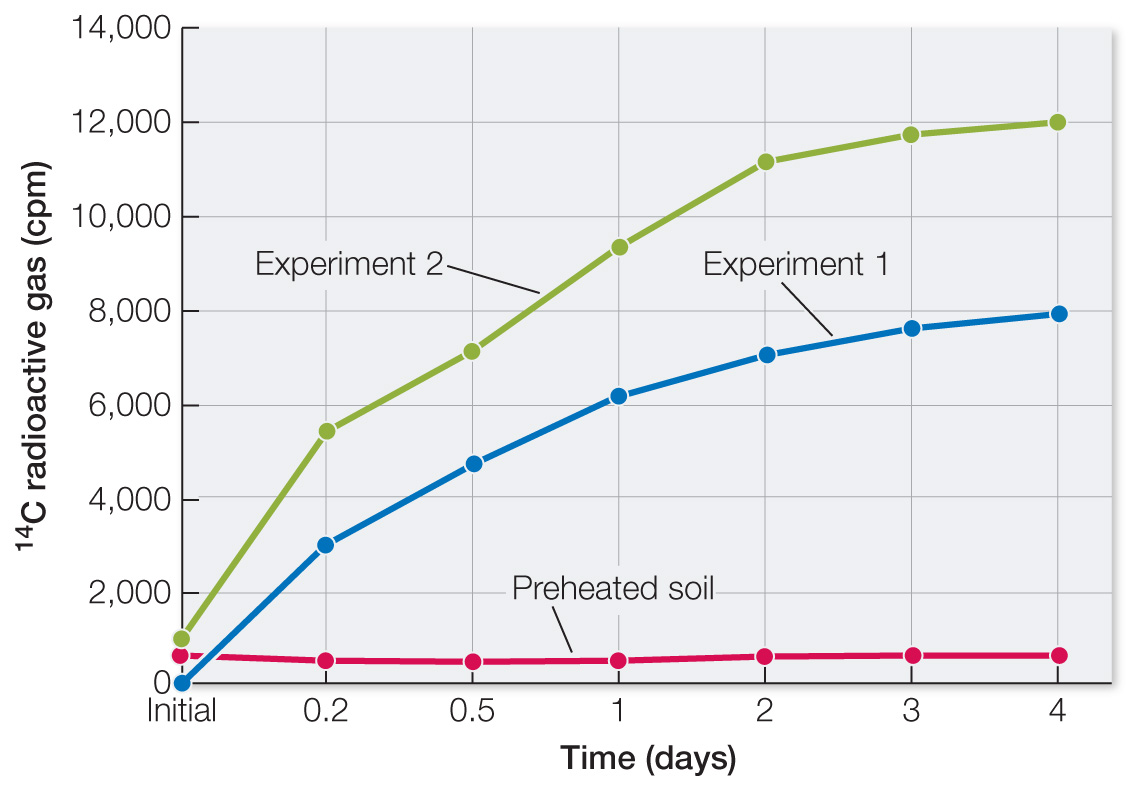
Question 3
Back on Earth, Ponnamperuma and colleagues simulated Martian soil by using a major component of Martian soil called hematite (Fe2O3) and added the nutrients in the same way as had been done in the Mars experiment. Their results, including a preheated experiment, are shown in Table B. How do these results affect the conclusions you drew after considering Questions 1 and 2?
The data for hematite are similar to the data for Martian soil. So while the Martian soil data are consistent with life, they are also consistent with non-
| 14C Gases Detected (counts per minute) | |||
|---|---|---|---|
| Time (days) | Experiment 1 | Experiment 2 | Preheated soil |
| Initial | 185 | 1,100 | 655 |
| 0.2 | 3,000 | 5,500 | 540 |
| 0.5 | 4,800 | 7,200 | 500 |
| 1.0 | 6,200 | 9,500 | 525 |
| 2.0 | 7,000 | 11,300 | 590 |
| 3.0 | 7,600 | 11,800 | 610 |
| 4.0 | 8,000 | 12,000 | 620 |
| 14C Gases Detected (counts per minute) | ||
|---|---|---|
| Hematite + 14C nutrients |
Hematite without nutrients |
|
| Experiment | 10,140 | 150 |
| Preheated 160°C | 308 | 107 |
A similar work with the data exercise may be assigned in LaunchPad.
75
A distinctive aspect of living systems is their ability to use molecules from the environment to extract chemical energy for growth. During this process, waste products are released. You are familiar with this: you breathe in oxygen gas (O2), take in nutrients in food, and breathe out carbon dioxide (CO2). Other organisms do not use O2 but take in other molecules. In the Mars experiments, scientists directed the Viking landers to scoop up some Martian soil and then exposed it to seven organic molecules as nutrients, all of which can be formed in Miller–
The results of some of the experiments were astonishing: radioactive gas was detected in some experiments, and some scientists concluded that there might be life on Mars. The fact that there was little radioactive gas produced when the soil was preheated to 160°C to kill living organisms gave credence to this conclusion. Later, simulations in the lab on Earth showed that radioactive gas could could be formed in this experiment with certain kinds of soils, without organisms. So there is an alternative, nonbiological explanation for the Mars data. Nevertheless, some biologists still adhere to the biological explanation for the Mars data, and the quest for life on Mars continues.