The cytoskeleton is important in cell structure and movement
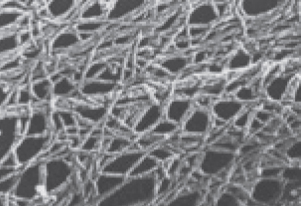 Cell shapes can sometimes change, and there appear to be rapid movements of structures inside cells. These phenomena involve a meshwork of rods within cells that can be seen in electron microscopy. Experimentation showed that this meshwork—
Cell shapes can sometimes change, and there appear to be rapid movements of structures inside cells. These phenomena involve a meshwork of rods within cells that can be seen in electron microscopy. Experimentation showed that this meshwork—
It supports the cell and maintains its shape.
It holds cell organelles and other particles in position within the cell.
It moves organelles and other particles around in the cell.
It is involved with movements of the cytoplasm, called cytoplasmic streaming.
It interacts with extracellular structures, helping anchor the cell in place.
investigating life
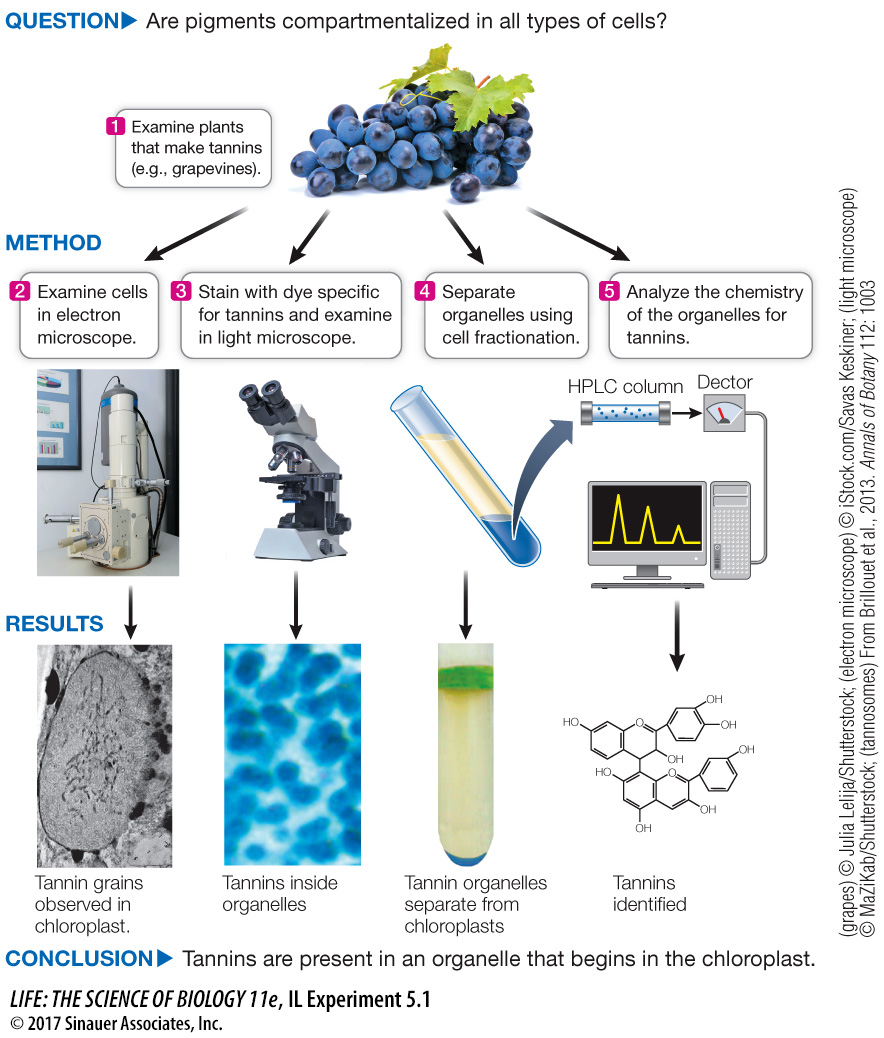
Discovering a New Organelle, the Tannosome
experiment
Original Paper: Brillouet, J.-M. et al. 2013. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Annals of Botany 112: 1003–
Through a series of investigations involving microscopy, cell fractionation, and chemical analysis, biologists discovered that plant tannins are compartmentalized in a heretofore undiscovered structure, the tannosome.
work with the data
The team of biologists and chemists (Brillouet et al., 2013) at the French National Institute for Agricultural Research, led by Geneviève Conejero, sought to investigate the cellular basis of tannin formation. It was well known from electron micrographs that these pigments accumulate in vacuoles, but how they got there was not established. The team used a combination of microscopy (light and electron) (see Figure 5.3), cell fractionation (see Figure 5.6), and chemical analysis to show that tannins are formed in discrete vesicles, which the researchers named tannosomes.
QUESTIONS
Question 1
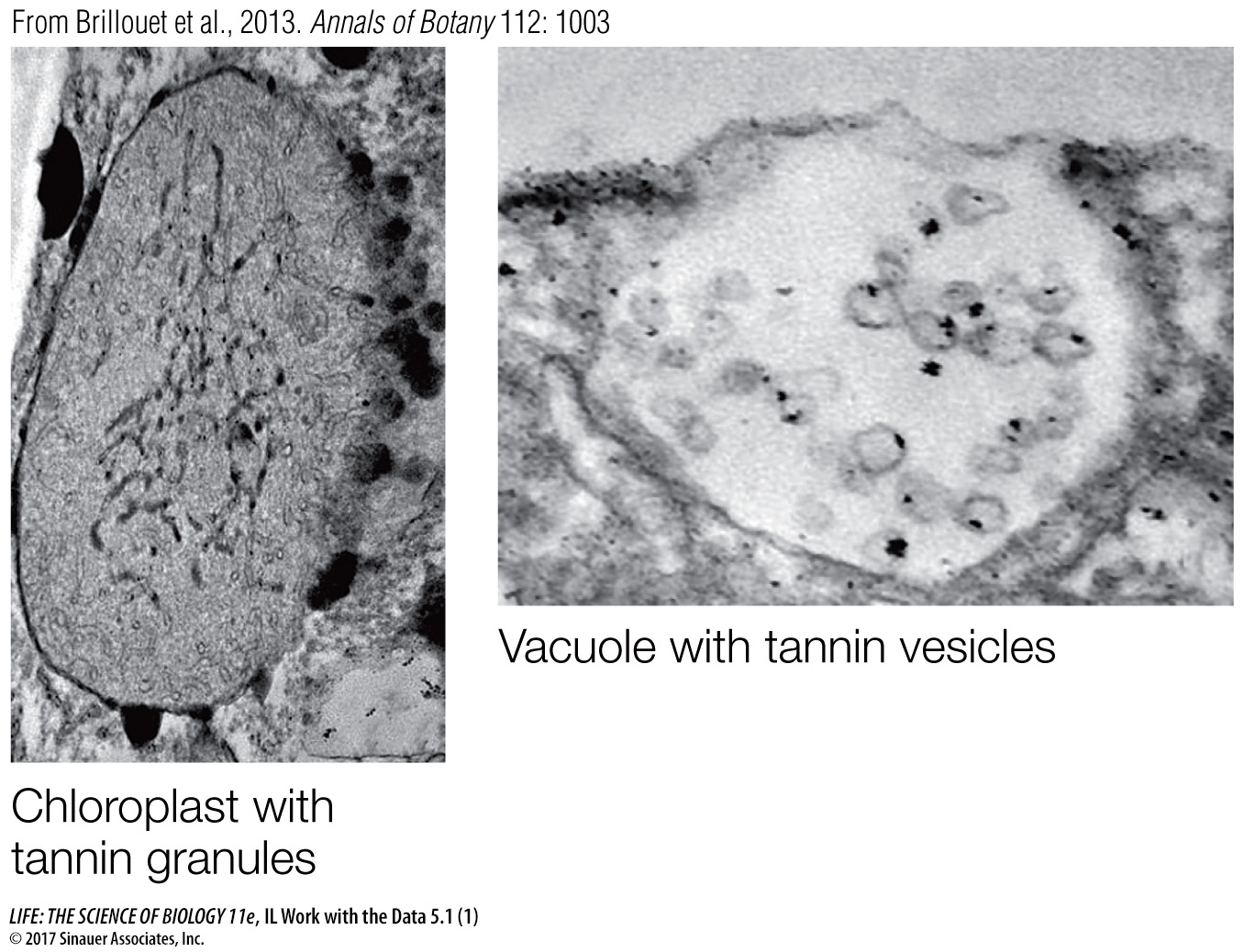
Plant organs rich in tannins were examined by electron microscopy. Representative photographs are shown in Figure A. What do these images indicate about the origin and final resting spot of tannins in the cell? Based on what you know of vesicle trafficking in the cell, how do you think the tannins get from the chloroplast to the vacuole?
The tannins appear in the chloroplast thylakoids and then are transferred to the vacuole by vesicles that enclose the tannins.
Figure A
Question 2
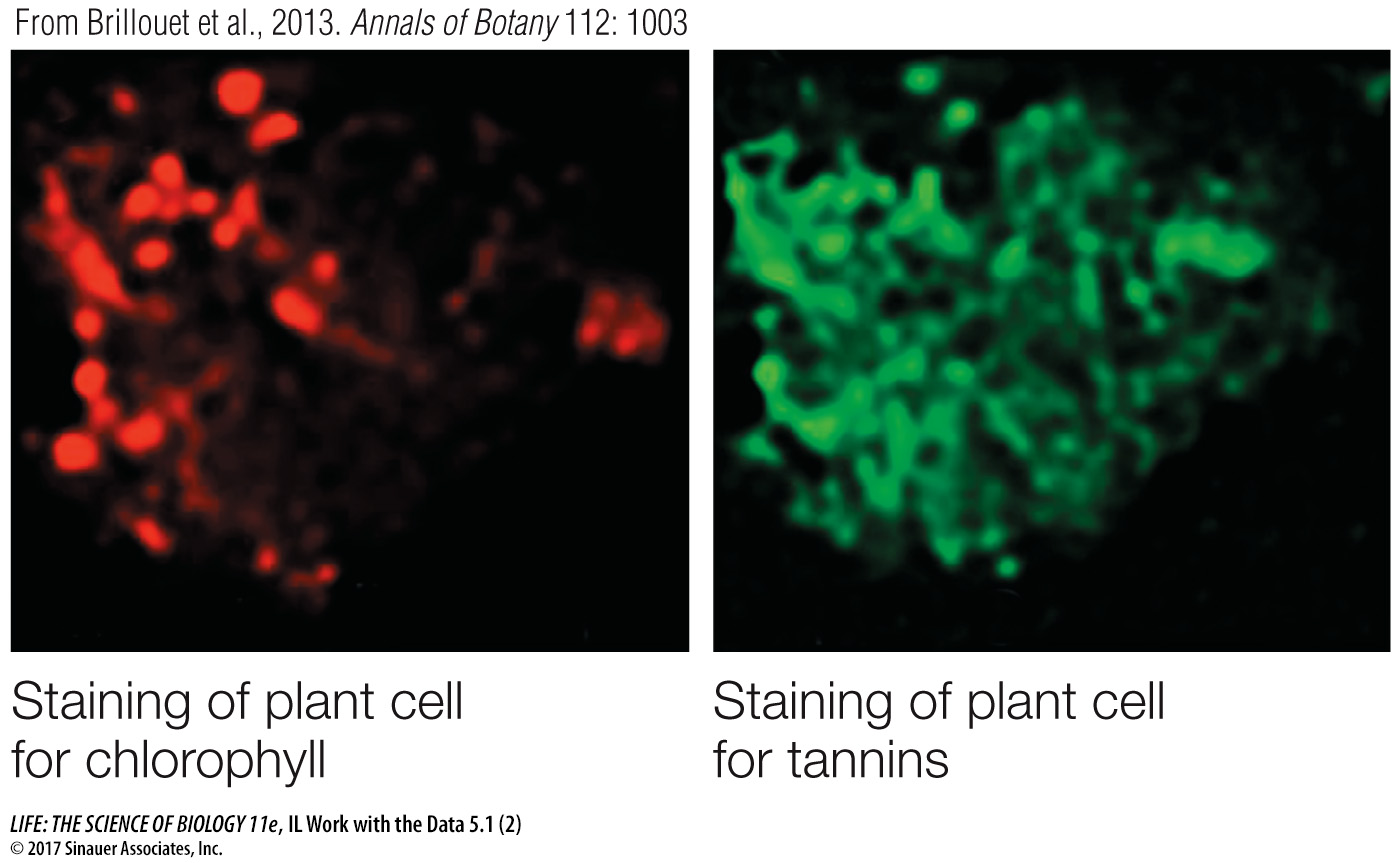
The scientists used specific dyes targeted to chlorophyll and tannins and examined slices of plant tissues by light microscopy. Figure B shows the results. What can you conclude about the subcellullar origin of tannins, and how does your answer relate to your answer to Question 1?
The staining for chlorophyll and tannins is in the same place. Since chlorophyll is in the chloroplast, this is consistent with a chloroplast origin for tannins, as described in the answer to Question 1.
Figure B
Question 3
Cell fractionation was used to try to isolate tannin-
Staining and chemical analysis both showed tannins, as well as cholorophyll, in the lower fraction of organelles, so this is probably a chloroplast fraction that contains tannins.
| Upper fraction | Lower fraction | |
|---|---|---|
| Staining for tannins | No | Yes |
| Staining for chlorophyll | Yes | Yes |
| Chemical analysis for tannins | No | Yes |
A similar work with the data exercise may be assigned in LaunchPad.
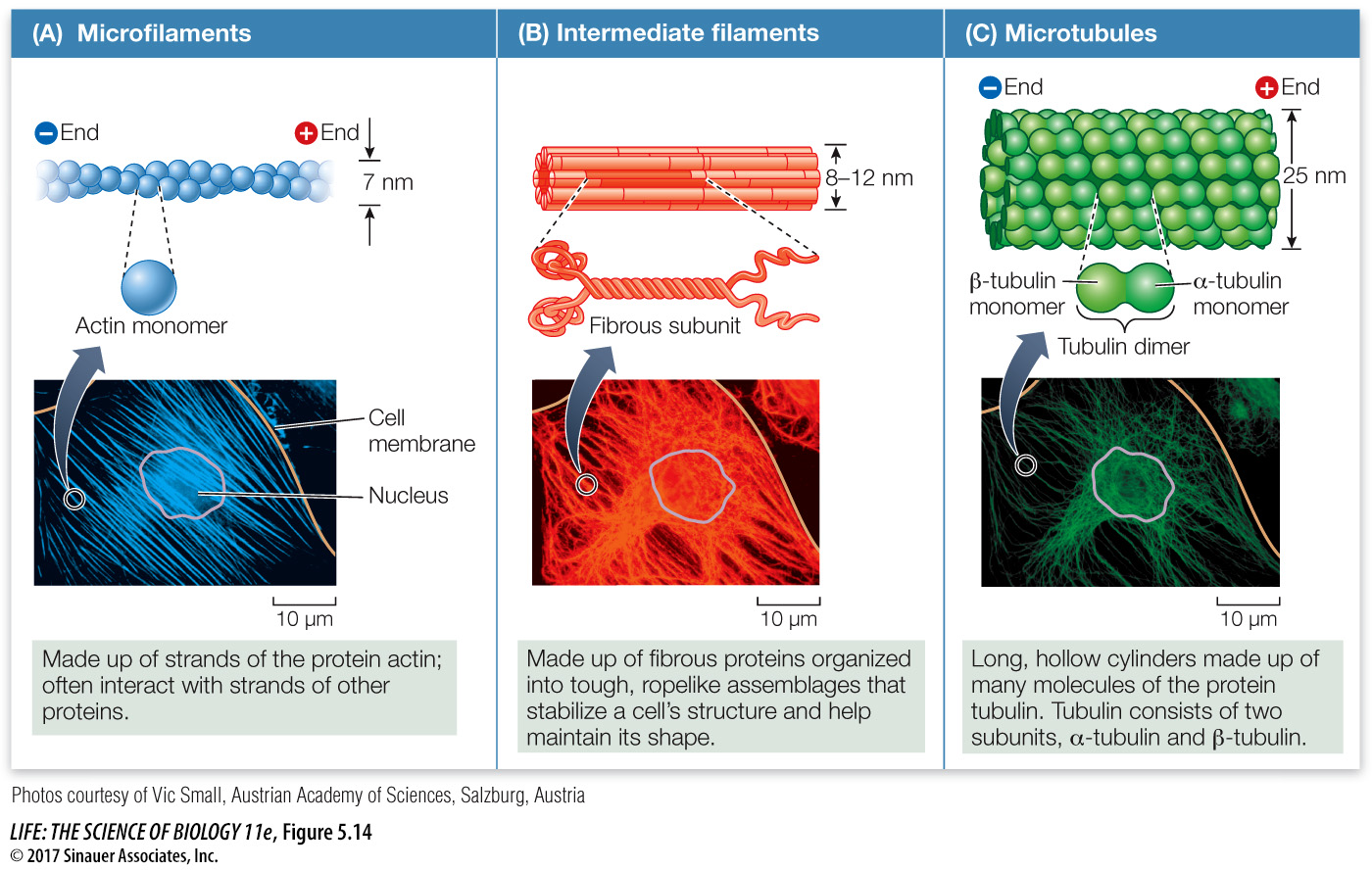
There are three components of the eukaryotic cytoskeleton: microfilaments (smallest diameter), intermediate filaments, and microtubules (largest diameter). These filaments have very different functions.
MICROFILAMENTS Microfilaments can exist as single filaments, in bundles, or in networks. They are about 7 nm in diameter and up to several micrometers long. Microfilaments have two major roles:
99
They help the entire cell or parts of the cell move.
They determine and stabilize cell shape.
Microfilaments are assembled from monomers of actin, a protein that exists in several forms and has many functions, especially in animals. The actin found in microfilaments (which are also known as *actin filaments) has distinct ends, designated “plus” and “minus.” These ends permit actin monomers to interact with one another to form long, double helical chains (Figure 5.14A). Within cells, the polymerization of actin into microfilaments is reversible, and the microfilaments can disappear from cells by breaking down into monomers of free actin. Special actin-
*connect the concepts In animal muscle cells, actin filaments are associated with another protein, the “motor protein” myosin, and the interactions of these two proteins account for the contraction of muscles, as described in Key Concept 47.1.
In non-
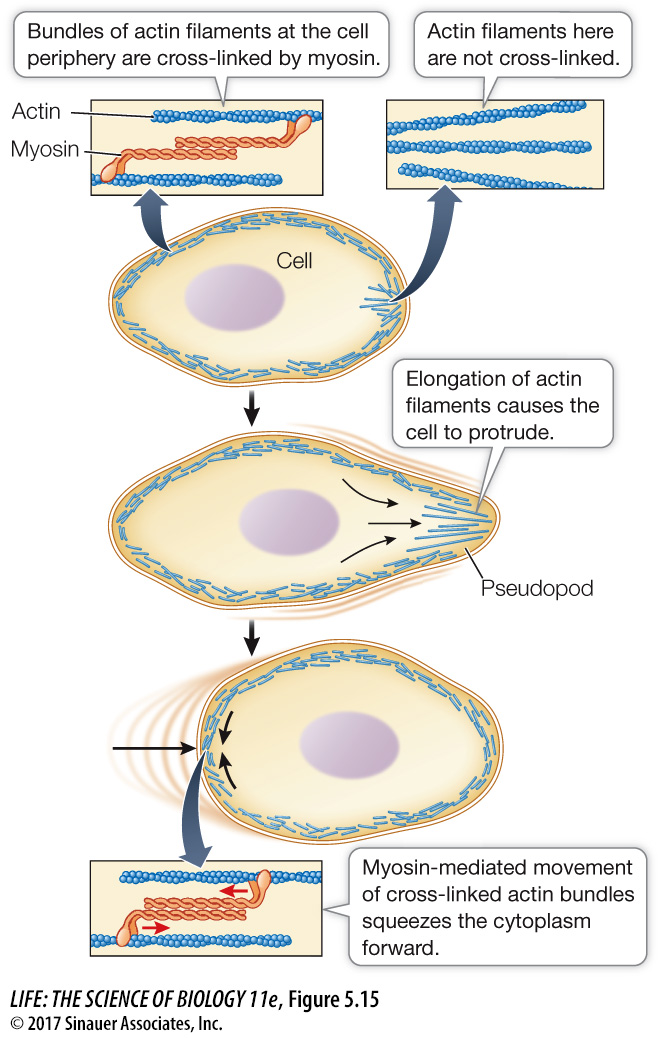
In some cell types, microfilaments form a meshwork just inside the cell membrane. Actin-
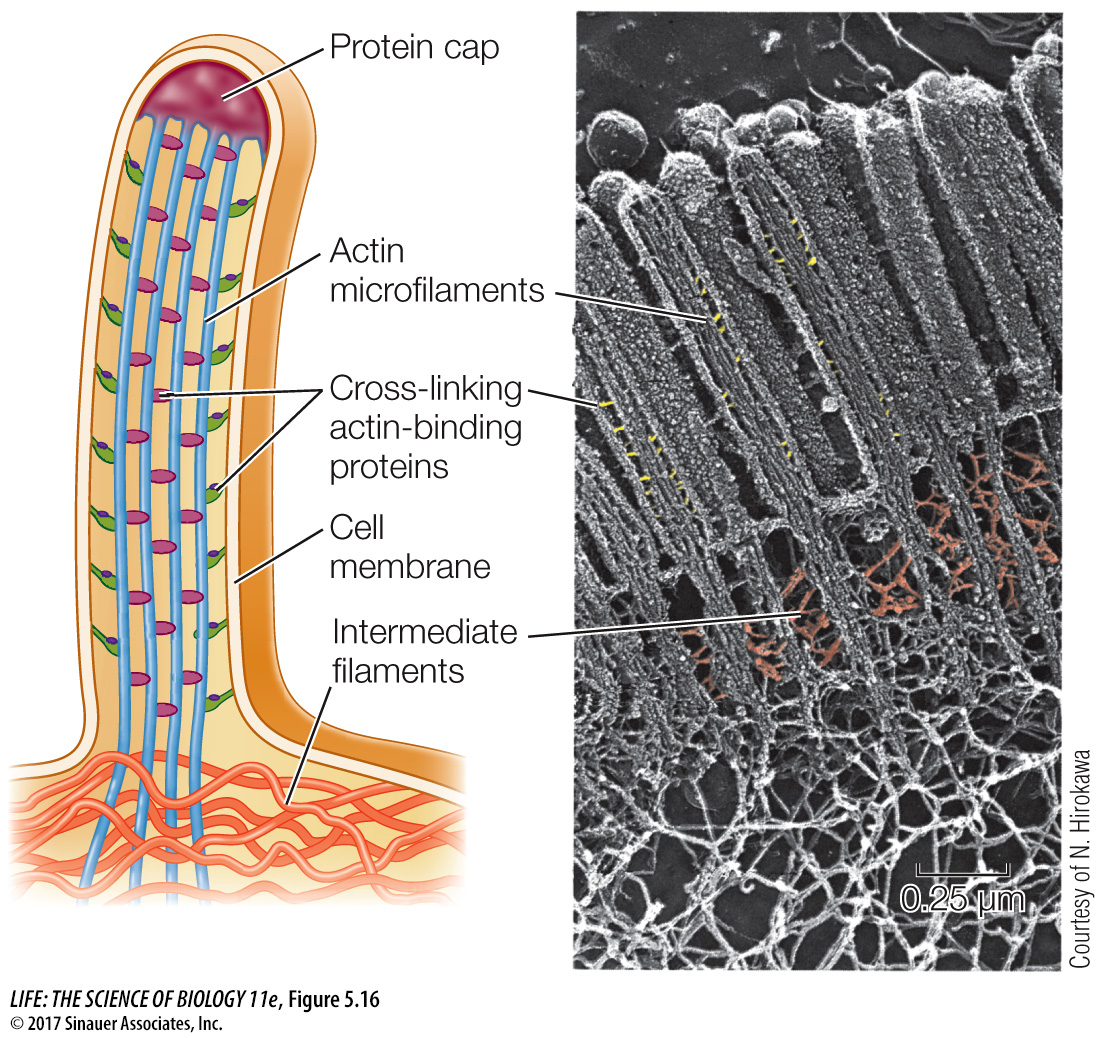
INTERMEDIATE FILAMENTS There are at least 50 different kinds of intermediate filaments, many of them specific to a few cell types. They generally fall into six molecular classes (based on amino acid sequence) that share the same general structure. One of these classes consists of fibrous proteins of the keratin family, which also includes the proteins that make up hair and fingernails. Intermediate filaments are tough, ropelike protein structures 8–
100
Intermediate filaments have two major structural functions:
They anchor cell structures in place. In some cells, intermediate filaments radiate from the nuclear envelope and help maintain the positions of the nucleus and other organelles in the cell. The lamins of the nuclear lamina are intermediate filaments. Other kinds of intermediate filaments help hold in place the complex apparatus of microfilaments in the microvilli of intestinal cells (see Figure 5.16).
They resist tension. For example, they maintain rigidity in body surface tissues by stretching through the cytoplasm and connecting specialized membrane structures called desmosomes (see Figure 6.7).
MICROTUBULES The largest-
They form a rigid internal skeleton for some cells.
They act as a framework along which motor proteins can move structures within the cell.
Microtubules are assembled from dimers–
α-tubulin + β-tubulin → doublet – polymer of doublets
(microtubule)
As in microfilaments, the two ends of a microtubule are different: one is designated the “plus” end and the other the “minus” end. Tubulin dimers can be rapidly added or subtracted, mainly at the plus end, lengthening or shortening the microtubule.
Many microtubules appear to radiate from a region of the cell called the microtubule organizing center. Tubulin polymerization results in a rigid structure, and tubulin depolymerization leads to its collapse.
Microtubule ↔ tubulin doublets
The capacity to change length rapidly makes microtubules dynamic structures: they are readily adapted for new purposes in the cell. For example, by disassembly and reassembly, microtubules can move to new parts of the cell and assemble new structures needed for cell division. Microtubules from all eukaryotes have this dynamic property, indicating that it is evolutionarily advantageous over a static, unchanging structure.
In plants, microtubules act as a framework for the assembly of cellulose and help orient the cellulose fibers of the cell wall (see Figure 3.18). Electron micrographs of plants frequently show microtubules lying just inside the cell membranes of cells that are forming or extending their cell walls. If the orientation of these microtubules is altered experimentally, it leads to a similar change in the cell wall and a new shape for the cell.
101
Microtubules serve as tracks for motor proteins, specialized molecules that use cellular energy to change their shapes and move. Motor proteins bind to and move along the microtubules, carrying materials from one part of the cell to another. Microtubules are also essential in distributing chromosomes to daughter cells during cell division. Because of this, drugs such as vincristine and taxol, which disrupt microtubule dynamics, also disrupt cell division. These drugs are useful for treating cancer, where cell division is excessive.
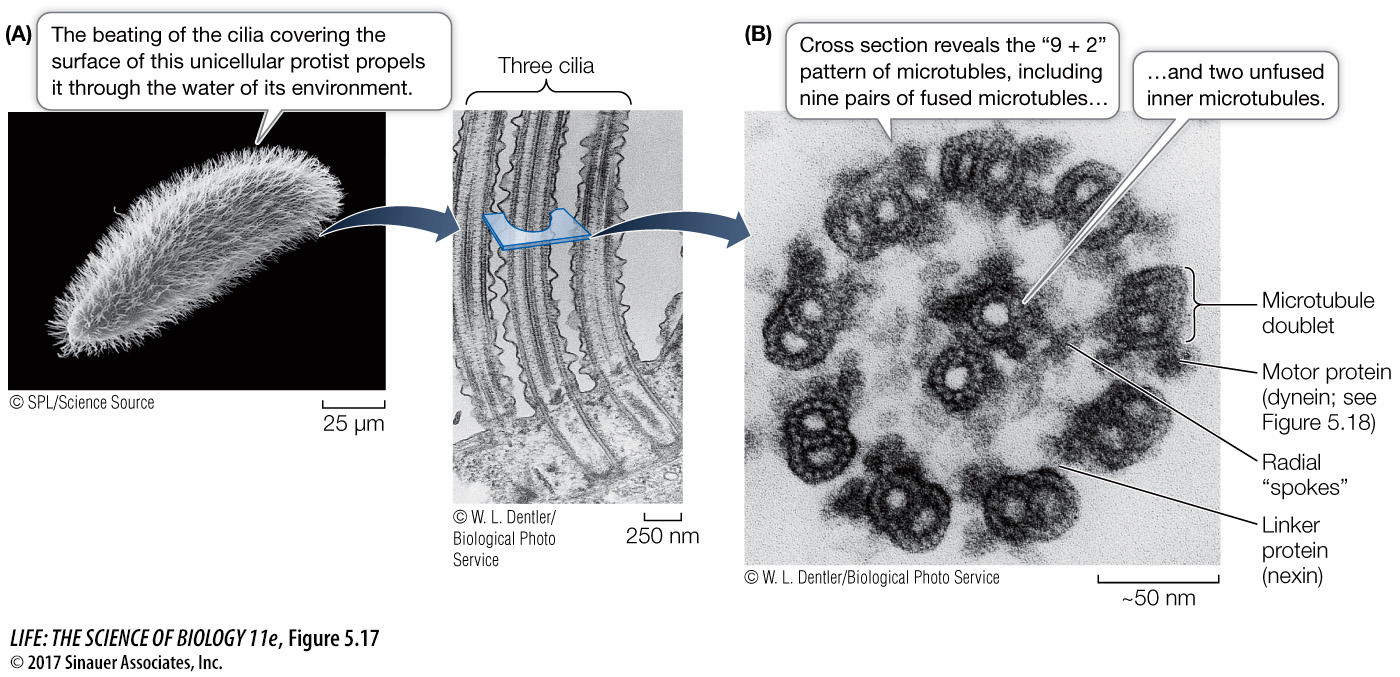
CILIA AND FLAGELLA Microtubules and their associated proteins line the interior of certain movable appendages on eukaryotic cells: the cilia (Figure 5.17A) and flagella. Many cells have one or the other of these appendages, which form from projections of the cell membrane:
102
Cilia are only 0.25 µm in length. They occur by the hundreds on individual cells and move stiffly to either propel the cell (for example, in protists) or to move fluid over a stationary cell (as in the human respiratory system).
Flagella are longer—
100 to 200 µm— and occur singly or in pairs. They can push or pull a cell through its aqueous environment.
In cross section, a typical cilium or eukaryotic flagellum is surrounded by the cell membrane and contains a “9 + 2” array of microtubules. As Figure 5.17B shows, nine fused pairs of microtubules form an outer cylinder, and one pair of unfused microtubules runs up the center. Each doublet is connected to the center of the structure by a radial spoke. This structure is essential to the bending motion of both cilia and flagella. How does this bending occur?
The motion of cilia and flagella results from the sliding of the microtubule doublets past one another. This sliding is driven by the motor protein dynein, which, like other motor proteins, works by undergoing reversible shape changes that require chemical energy. Dynein molecules bind between two neighboring microtubule pairs, and as the dynein molecules change shape, the pairs move past one another (Figure 5.18). Another protein, nexin, cross-
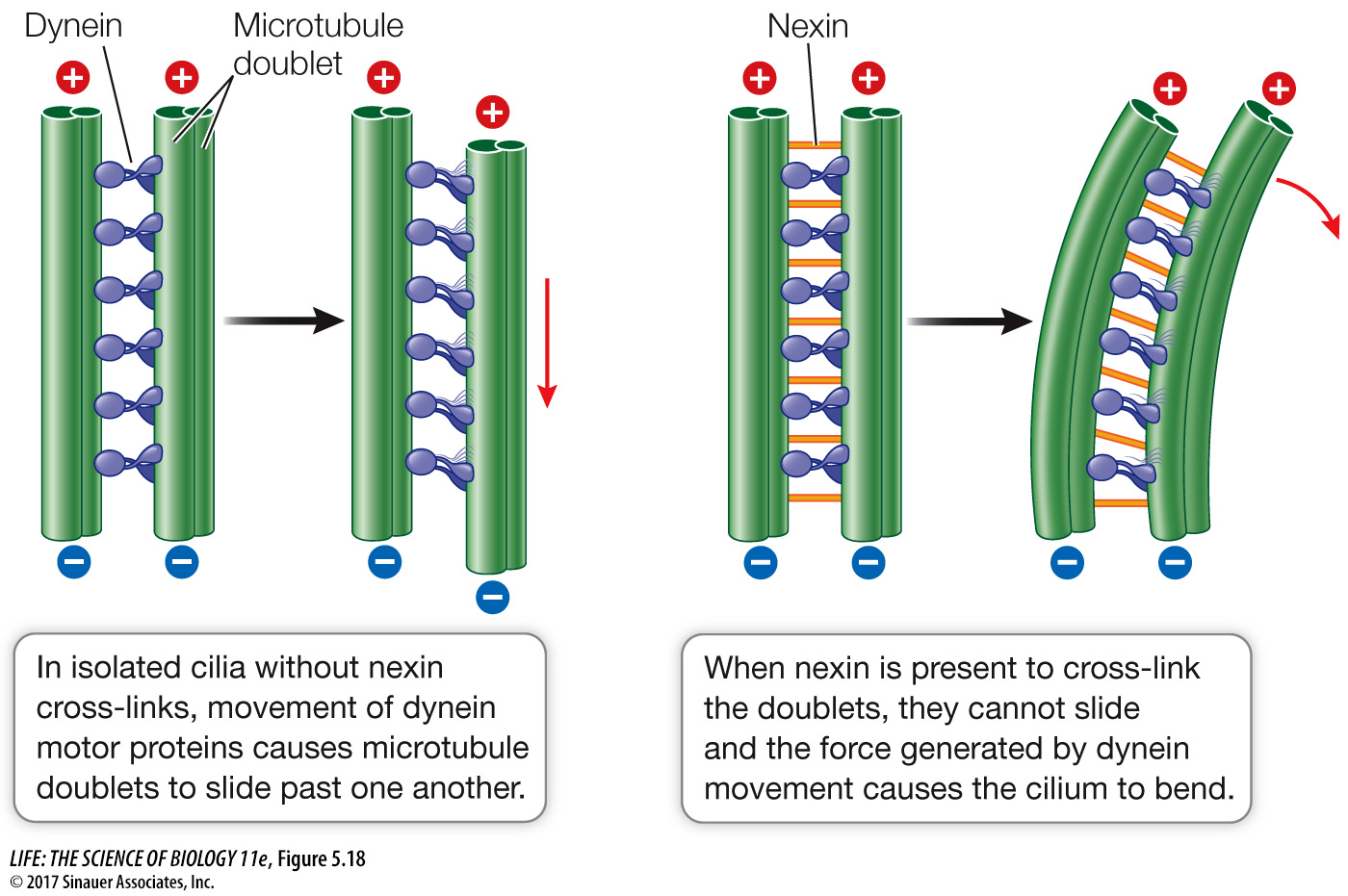
Question
Q: In some people, the nexin links are absent and microtubule doublets are not attached to one another. What do you think the consequences of this would be?
Nexin links cause cilia and flagella to bend when microtubule doublets try to slide past one another. Absence of nexin would result in reduced flagella and ciliary function. This is called immotile cilia syndrome.
Other motor proteins, including kinesin, carry protein-
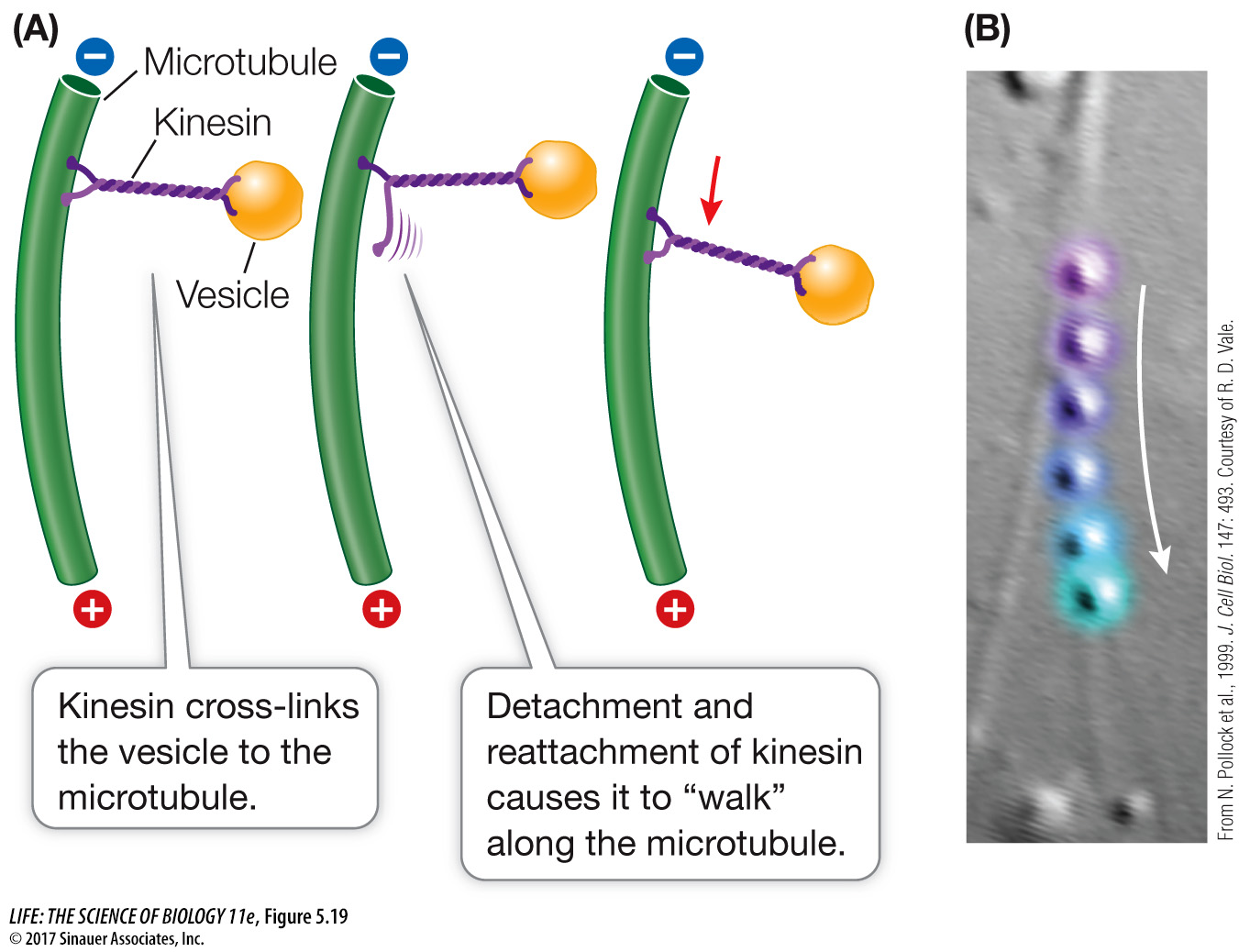
*connect the concepts Perhaps the most dramatic role of microtubules is in the process of chromosome movement during cell division. See Key Concept 11.3.