Diffusion is the process of random movement toward a state of equilibrium
In a solution, there is a tendency for all of the components to be evenly distributed. You can see this when a drop of food coloring falls into a glass of water. Initially the pigment molecules are very concentrated, but they will move about at random, slowly spreading until the intensity of the color is eventually the same throughout the glass.
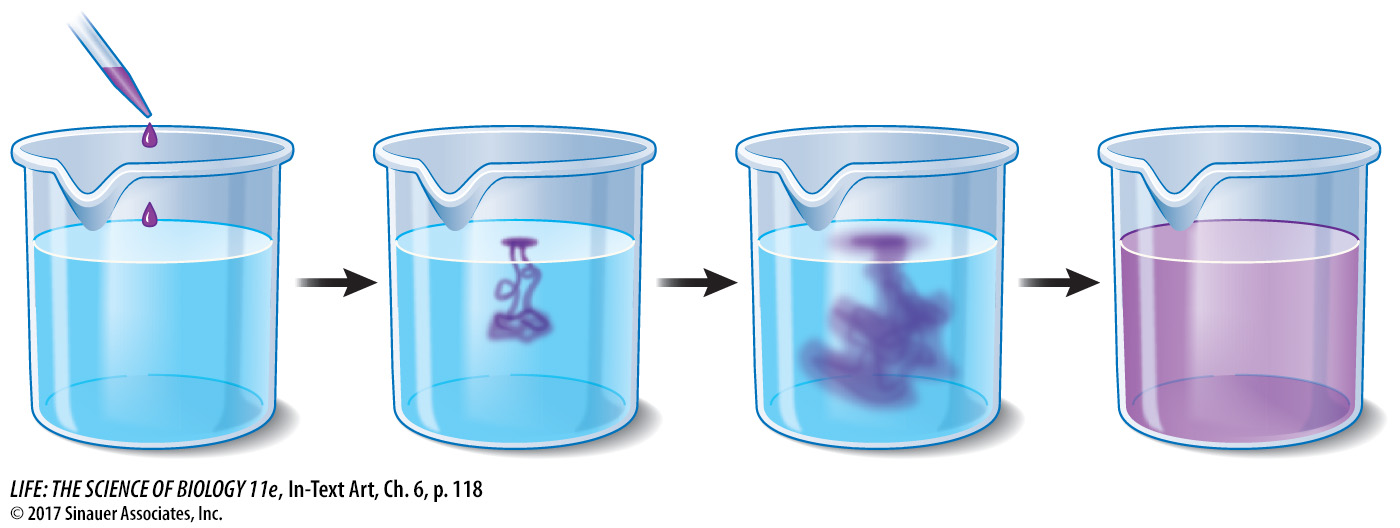
A solution in which the solute particles are uniformly distributed is said to be at equilibrium because there will be no future net change in their concentration. Being at equilibrium does not mean that the particles have stopped moving; it just means that they are moving in such a way that their overall distribution does not change.
Diffusion is the process of random movement toward a state of equilibrium. Although the motion of each individual particle is absolutely random, the net movement of particles is directional until equilibrium is reached. Diffusion is thus a net movement from regions of greater concentration to regions of lesser concentration. In a solution with many different substances dissolved in it (solutes), the diffusion of each solute is independent of those of the others. How fast a substance diffuses depends on four factors:
The size and mass of the molecules or ions: smaller molecules diffuse faster.
The temperature of the solution: higher temperatures lead to faster diffusion because ions or molecules have more energy, and thus move more rapidly, at higher temperatures.
The density of the solution: As the density of the solution through which a substance is diffusing increases, the diffusion rate decreases.
The concentration gradient in the system—
that is, the change in solute concentration with distance in a given direction: the greater the concentration gradient, the more rapidly a substance diffuses.
119
In our example shown above, the gradient is the concentration of food coloring in the droplet when first dropped into the water as compared with the concentration of food coloring in the water on the edge of the glass. When the food coloring is first dropped into the water, there is a high (or steep) concentration gradient. As time passes and the coloring diffuses through the solution, the concentration gradient slowly decreases.
DIFFUSION WITHIN CELLS AND TISSUES In a small volume such as that inside a cell, solutes distribute themselves rapidly by diffusion. Small molecules and ions may move from one end of a cell to another in a millisecond (10–3 s, or one-
DIFFUSION ACROSS MEMBRANES In a solution without barriers, all the solutes diffuse at rates determined by temperature, their physical properties, their concentration gradients, and the density of the solution. If a biological membrane divides the solution into separate compartments, then the movement of the different solutes can be affected by the properties of the membrane. The membrane is said to be permeable to solutes that can cross it more or less easily, but impermeable to substances that cannot move across it.
Molecules to which the membrane is impermeable remain in separate compartments, and their concentrations may be different on the two sides of the membrane. Molecules to which the membrane is permeable diffuse from one compartment to the other until their concentrations are equal on both sides of the membrane, and equilibrium is reached. After that point, individual molecules will continue to pass through the membrane, but there will be no net change in concentration.