Osmosis is the diffusion of water across membranes
Water molecules pass through specialized channels in membranes (see the opening story) by a diffusion process called *osmosis. This completely passive process uses no metabolic energy and depends on the relative concentrations of the water molecules on each side of the membrane. It is important that you remember that in a particular solution, the higher the total solute concentration, the lower the concentration of water molecules. A membrane may allow water but not solutes to pass across it, and in that case, water will diffuse across the membrane toward the side with the higher solute (lower water) concentration.
*connect the concepts Osmosis plays key roles in plant physiology (e.g., in the root; see Key Concept 34.1) and in animal physiology (e.g., in the kidney; see Key Concept 51.1).
Three terms are used to compare the solute concentrations of two solutions separated by a membrane:
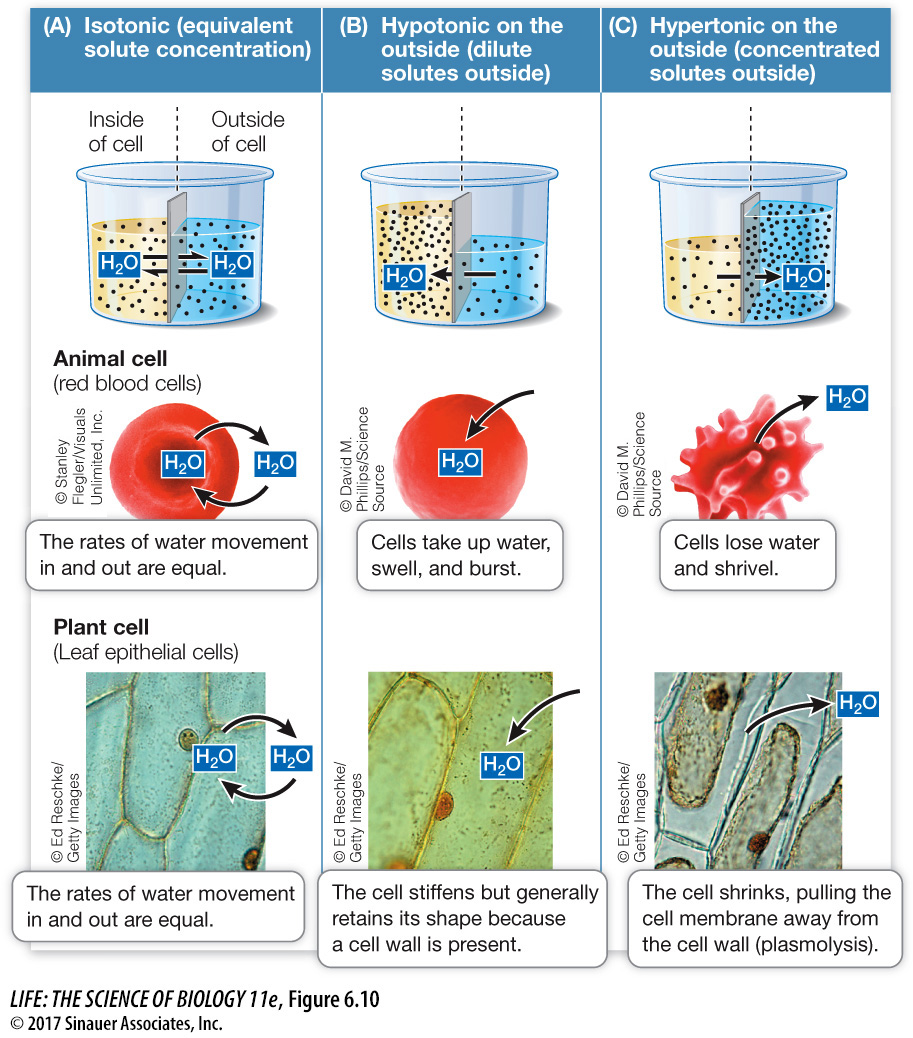
Isotonic solutions have equal solute concentrations (Figure 6.10A).
A hypotonic solution has a lower solute concentration than the other solution with which it is being compared (Figure 6.10B).
A hypertonic solution has a higher solute concentration than the other solution with which it is being compared (Figure 6.10C).
Question
Q: If you overfertilize the soil of a potted plant, the plant often wilts. Why?
Overfertilizing makes the soil water hypertonic to the interior of the plant root cells. Water leaves the plant roots by osmosis, moving toward the hypertonic medium. Water in the plant organs also travels to the roots, where it leaves by osmosis. Because cellular water is important in maintaining turgor pressure, the loss of water resulting from overfertilization causes the plant to wilt.
In general, when we discuss tonicity in cells, we refer to the outside solution in comparison to the inside of the cell. Therefore a solution that has a higher concentration of solutes than the concentration inside the cell is hypertonic. To reach equilibrium, water would move out of the cell into the surrounding solution. Water moves from a hypotonic solution across a membrane to a hypertonic solution.
120
When we say that “water moves,” bear in mind that we are referring to the net movement of water. Since it is so abundant, water is constantly moving through protein channels across the cell membrane into and out of cells. What concerns us here is whether the overall movement is greater in one direction or the other.
The concentration of solutes in the environment determines the direction of osmosis in all animal cells. A red blood cell takes up water from a solution that is hypotonic to the cell’s contents. The cell bursts because its cell membrane cannot withstand the pressure created by the water entry and the resultant swelling. Conversely, the cell shrinks if the solution surrounding it is hypertonic to its contents. The integrity of red and white blood cells is absolutely dependent on the maintenance of a constant solute concentration in the blood plasma: the plasma must be isotonic to the blood cells if the cells are not to burst or shrink. Regulation of the solute concentration of body fluids is thus an important process for organisms without cell walls. Aquatic invertebrates usually have internal solute concentrations that match those of their aqueous environment. However, fish often have a very different internal environment: for example, the environment around a fish living in a freshwater stream is hypotonic to the body fluids inside the fish. Considerable chemical energy must be used to maintain this imbalance.
Unlike animal cells, the cells of plants, archaea, bacteria, fungi, and some protists have cell walls outside of their cell membrane that limit their volumes and keep them from bursting. Cells with sturdy walls take up a limited amount of water, and in so doing they build up internal pressure against the cell wall, which prevents further water from entering. This pressure within the cell is called turgor pressure. Turgor pressure keeps plants upright (and lettuce crisp) and is the driving force for the enlargement of plant cells. It is a normal and essential component of plant growth. If enough water leaves the cells, turgor pressure drops and the plant wilts. Turgor pressure reaches about 100 pounds per square inch (0.7 kg/cm2)—several times greater than the pressure in automobile tires. This pressure is so great that the cells would change shape and detach from one another were it not for adhesive molecules in the plant cell walls and the plasmodesmata linking the cell cytoplasms (see Key Concept 5.4).