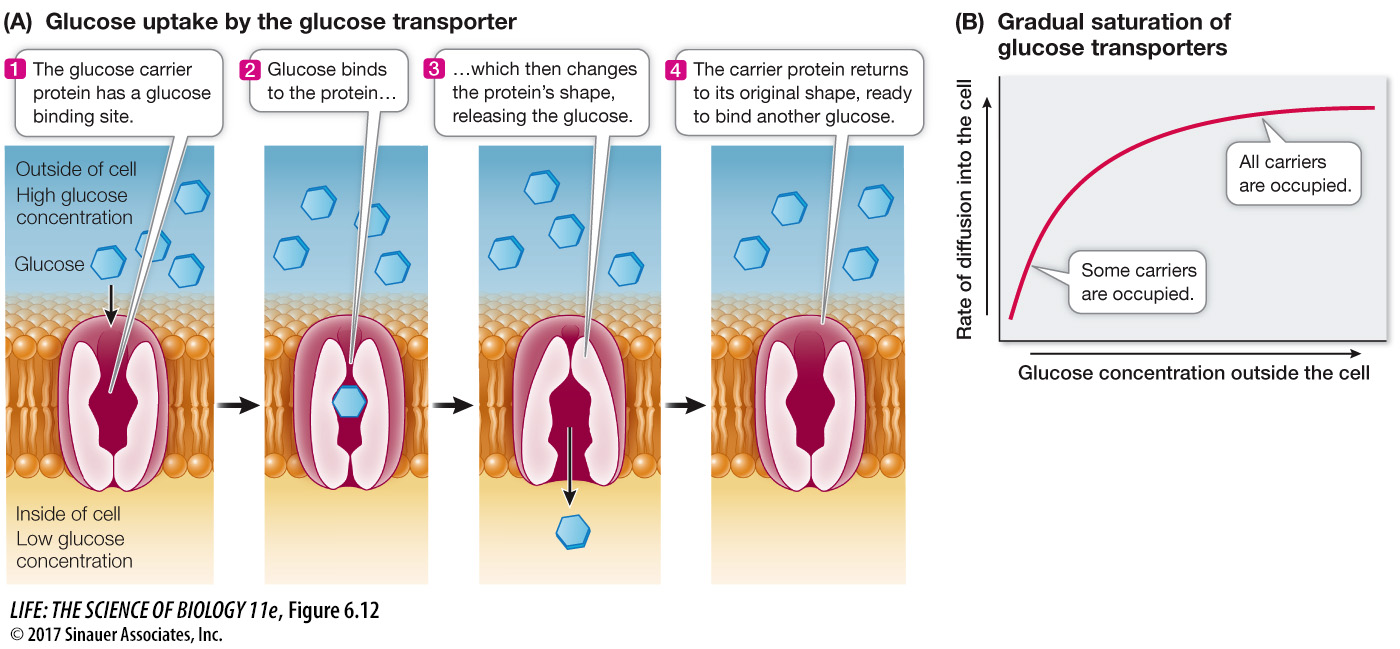
Carrier proteins aid diffusion by binding substances
As we mentioned earlier, another type of facilitated diffusion involves the binding of the transported substance to a membrane protein called a carrier protein. Like channel proteins, carrier proteins facilitate the passive diffusion of substances into or out of cells or organelles. Carrier proteins transport polar molecules such as sugars and amino acids.
Glucose is the major energy source for most cells, and living systems require a great deal of it. Glucose is polar and cannot readily diffuse across membranes. Eukaryotic cell membranes contain a carrier protein—
Activity 6.4 Membrane Transport Simulation
www.life11e.com/
Transport by carrier proteins is different from simple diffusion. In simple diffusion, the rate of movement depends on the concentration gradient across the membrane. This is also true for carrier-
The rate of diffusion reaches a maximum when all the carrier molecules are fully loaded with solute molecules. Think of waiting for the elevator on the ground floor of a hotel with 50 other people. You can’t all get in the elevator (carrier) at once, so the rate of transport (say, ten people at a time) is at its maximum, and the transport system is “saturated.” As a consequence, cells that require large amounts of energy, such as muscle cells, have high concentrations of glucose transporters in their membranes so that the maximum rate of facilitated diffusion is greater. Likewise, the human brain has high glucose needs, and the blood vessels that nourish it have high concentrations of glucose transporters.
122
investigating life
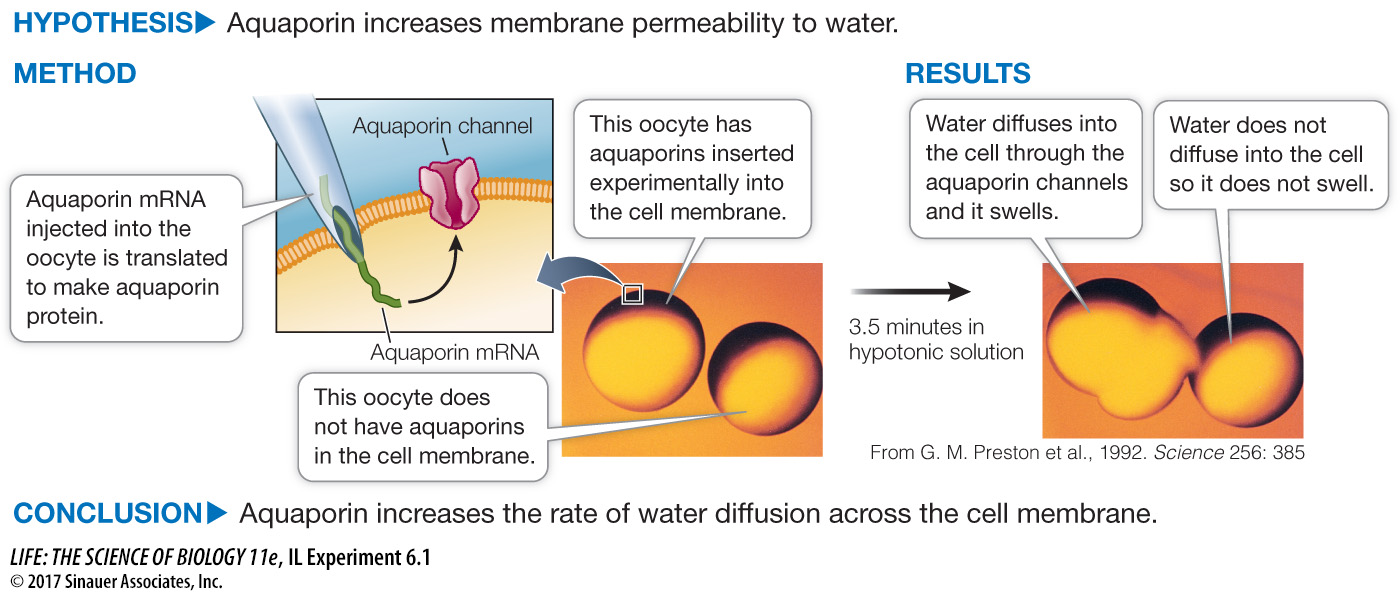
Aquaporins Increase Membrane Permeability to Water
experiment
Original Paper: Preston, G. M., T. P. Carroll, W. B. Gugino and P. Agre. 1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256: 385–
A protein was isolated from the membranes of cells in which water diffuses rapidly across the membranes. When the protein was inserted into oocytes, which do not normally have it, the water permeability of the oocytes was greatly increased.
work with the data
Although diffusion can account for limited water movement across cell membranes, simple diffusion seemed unlikely to explain the considerable water movement in kidney and red blood cells. By chance, Peter Agre and his colleagues found a major membrane protein, CHIP28, shared by these two cell types, and hypothesized that it was responsible for cell membrane water transport. So they did a “what if” experiment, taking the mRNA for CHIP28 and injecting it into frog oocytes that do not normally make the protein.
QUESTIONS
Question 1
Frog oocytes in isotonic liquid were injected with either a small amount of water (control) or with CHIP28 mRNA in a small amount of water. The oocytes were then transferred to a hypotonic medium, and changes in relative cell volume were measured by microscopy. Table A shows the results.
| Cell volume | ||
|---|---|---|
| Time (min) | CHIP28 mRNA | Water only (control) |
| 0 | 1.0 | 1.0 |
| 0.5 | 1.05 | 1.0 |
| 1 | 1.15 | 1.02 |
| 1.5 | 1.23 | — |
| 2 | 1.32 | 1.02 |
| 2.5 | 1.36 | — |
| 3 | 1.41 | 1.02 |
| 4 | (burst) | 1.03 |
Plot the data on a graph of relative cell volume versus time after injection. How do the mRNA and control eggs compare? What explains the increase in the volume of the mRNA-
The mRNA-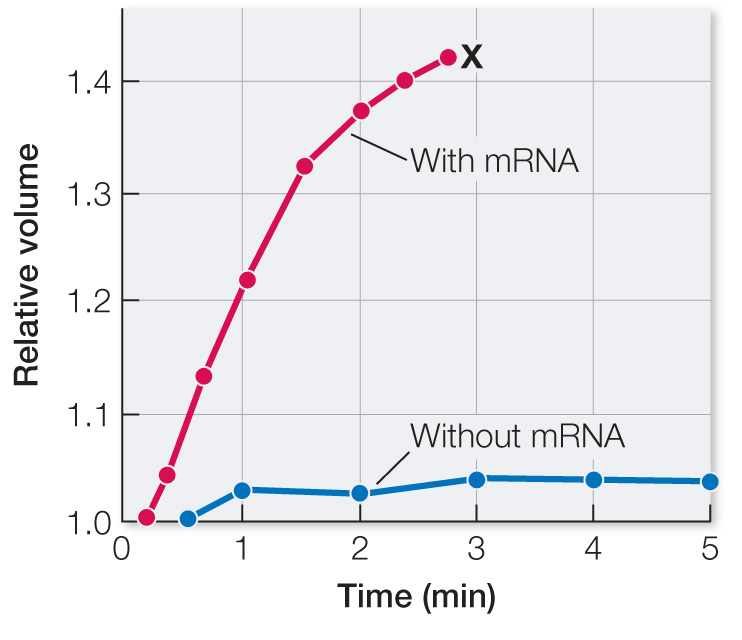
Question 2
Water permeability (Pf) was calculated by the rate of osmotic swelling. Table B shows the results when increasing amounts of CHIP28 mRNA were injected.
| Amount of mRNA (ng) | Pf (cm/sec × 10–4)a |
|---|---|
| 0 | 13.7 (3.3) |
| 0.1 | 50.0 (10.1) |
| 0.5 | 112 (29.2) |
| 2.0 | 175 (38.4) |
| 10.0 | 221 (14.8) |
a Numbers in parentheses indicate +/– standard deviation.
What can you conclude from these data? What statistical test (see Appendix B) would you do to show the significance of your conclusion?
Water permeability increased with more mRNA injected, presumably because there was more aquaporin in the membranes that had more mRNA. The relationship could be evaluated statistically by linear regression.
Question 3
To further investigate the role of CHIP28 in water transport, oocytes were given a known inhibitor of protein-
| mRNA injected | Mercuric chloride |
Mercapto- ethanol |
Pf (cm/sec × 10–4) |
|---|---|---|---|
| None | None | None | 27.9 |
| None | Yes | None | 20.3 |
| None | Yes | Yes | 25.4 |
| CHIP28 | None | None | 210 |
| CHIP28 | Yes | None | 80.7 |
| CHIP28 | Yes | Yes | 188 |
What can you conclude about the molecular nature of water transport mediated by CHIP28 mRNA? What data support your conclusion? Explain all the controls that were done.
The data on the mRNA-
A similar work with the data exercise may be assigned in LaunchPad.