Second messengers can amplify signals between receptors and target molecules
Often there is a small molecule intermediary between the activated receptor and the cascade of events that ensues. Earl Sutherland and his colleagues at Case Western Reserve University discovered one such molecule when they were investigating the activation of the liver enzyme glycogen phosphorylase by the hormone epinephrine. The hormone is released when an animal faces life-
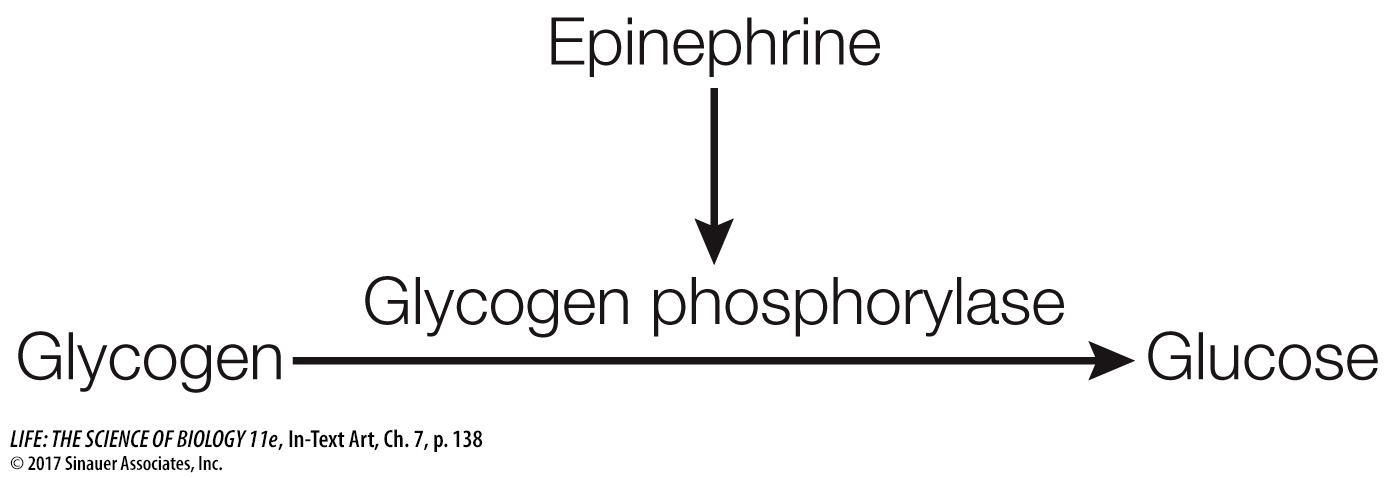
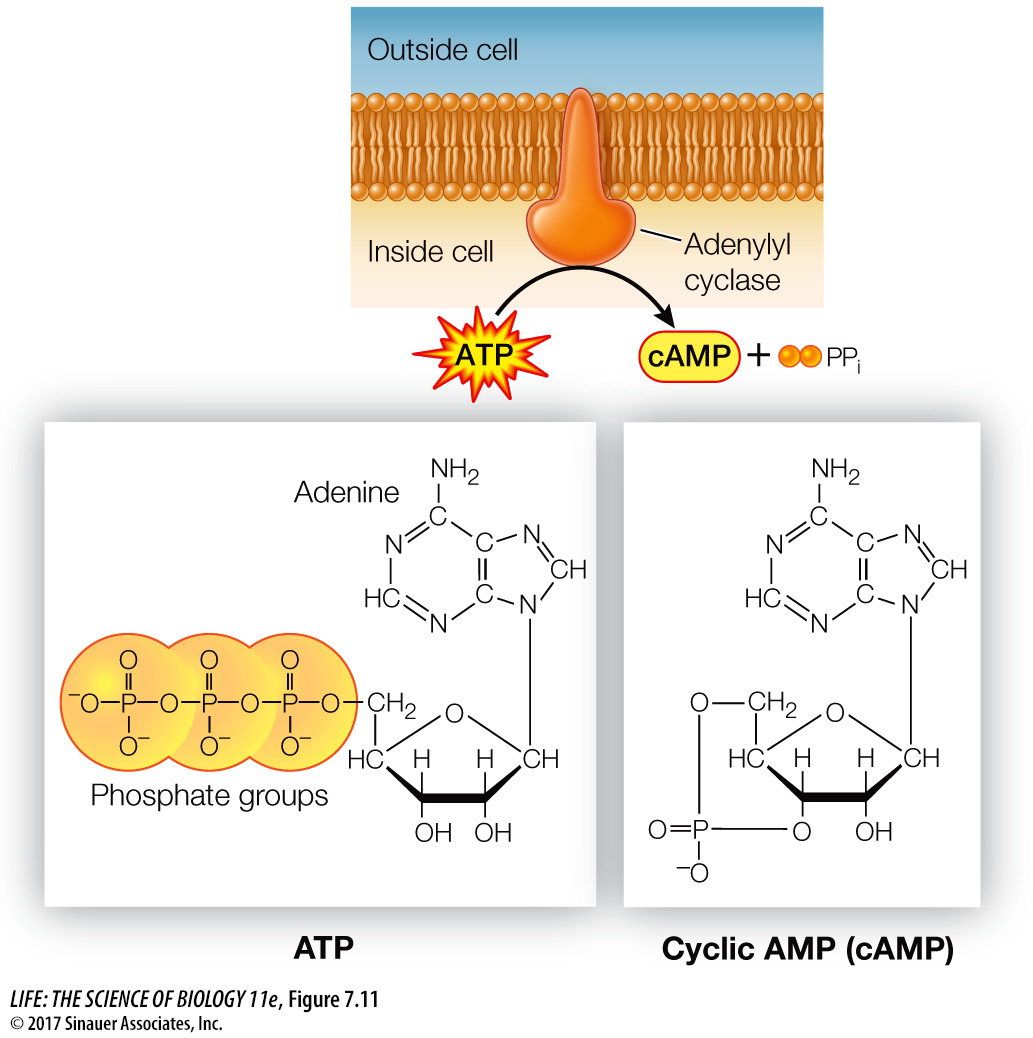
The researchers found that epinephrine could activate glycogen phosphorylase in liver cells that had been broken open, but only if the entire cell contents, including cell membrane fragments, were present. Under these conditions epinephrine was bound to the cell membrane fragments (the location of its receptor), but the active phosphorylase was present in the solution. Adding epinephrine to just cytoplasm with inactive phosphorylase did not result in activation. The researchers hypothesized that there must be a “second messenger” that transmits the epinephrine signal (epinephrine being the “first messenger”). The experiments confirmed the existence of a second messenger, later identified as cyclic AMP (cAMP), which is produced from ATP by the enzyme adenylyl cyclase (Figure 7.11). Adenylyl cyclase is activated via a G protein-
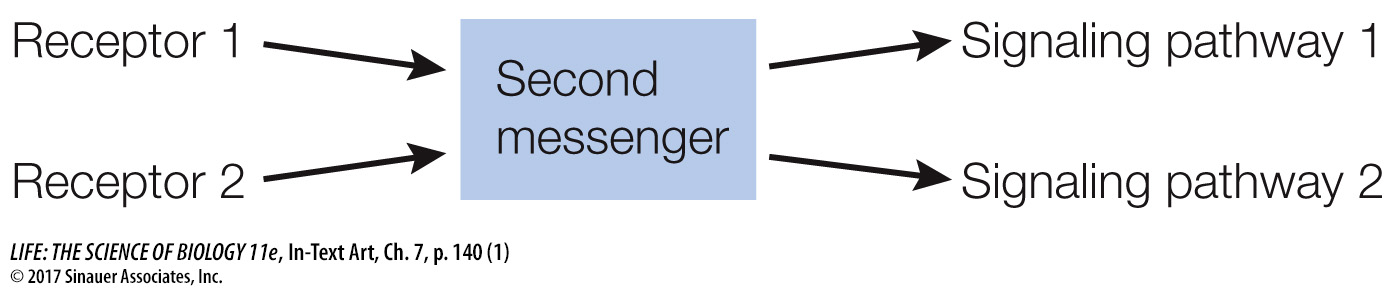
In contrast to the specificity of receptor binding, second messengers such as cAMP allow a cell to respond to a single event at the cell membrane with many events inside the cell. Thus second messengers serve to rapidly amplify and distribute the signal—
Second messengers are often involved in crosstalk between different signaling pathways. Activation of the epinephrine receptor is not the only way for a cell to produce cAMP; and as noted, there are multiple targets of cAMP in the cell, and these targets are parts of other pathways.
140
Several other classes of second messengers have since been identified, including lipid-
LIPID-
The best-
As with cAMP, the receptors involved in this second-
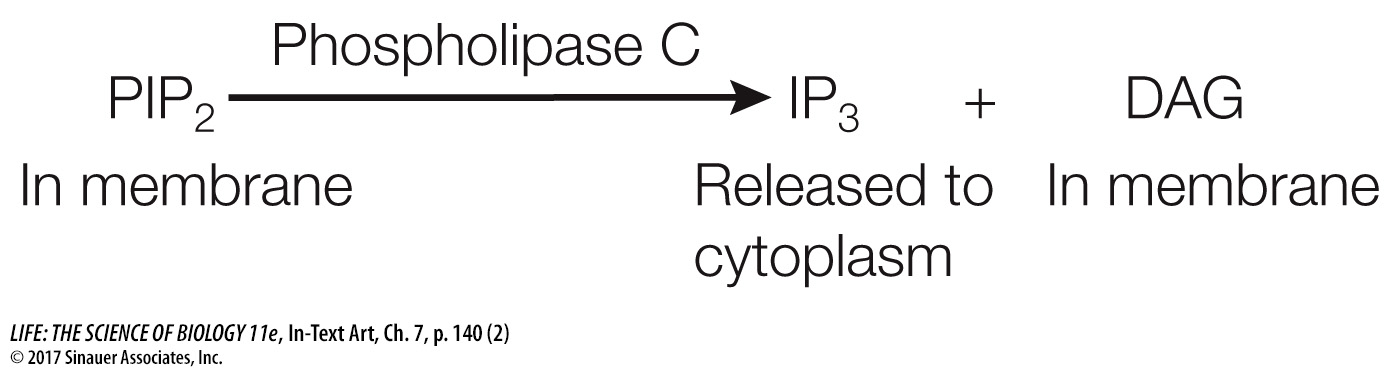
IP3 and DAG are both second messengers; they have different modes of action that build on each other to activate protein kinase C (PKC). PKC refers to a family of protein kinases that can phosphorylate a wide variety of target proteins, leading to a multiplicity of cellular responses that vary depending on the tissue or cell type.
CALCIUM IONS Calcium ions (Ca2+) are scarce inside most cells, which have cytosolic Ca2+ concentrations of only about 0.1 micromolar (μM). Ca2+ concentrations outside cells and within the endoplasmic reticulum (ER) are usually much higher. Active transport proteins in the cell and ER membranes maintain this concentration difference by pumping Ca2+ out of the cytosol. In contrast to cAMP and the lipid-
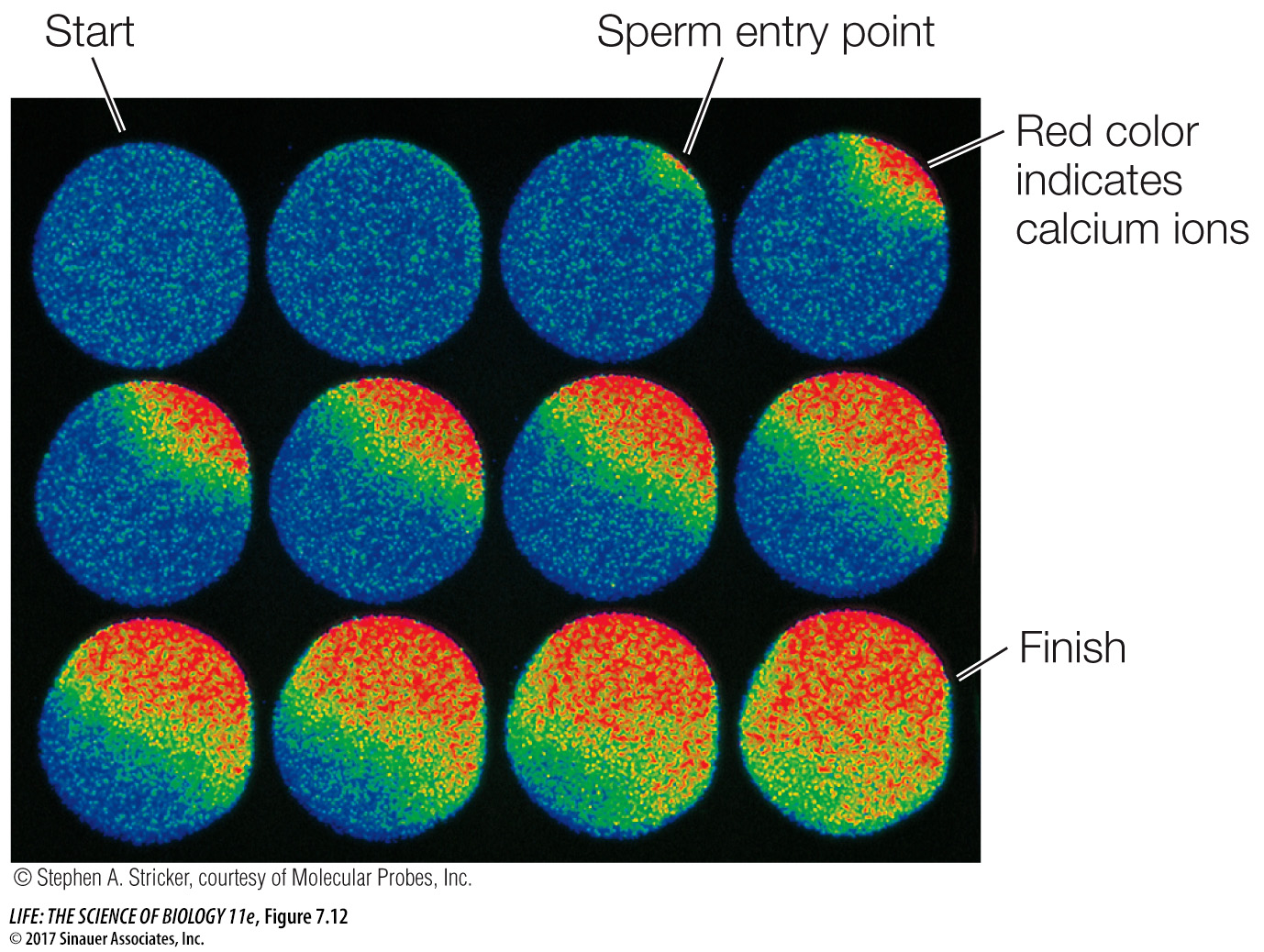
Many signals can cause calcium channels to open, including IP3. The entry of a sperm into an egg is a very important signal that causes a massive opening of calcium channels, resulting in numerous and dramatic changes that prepare the now-
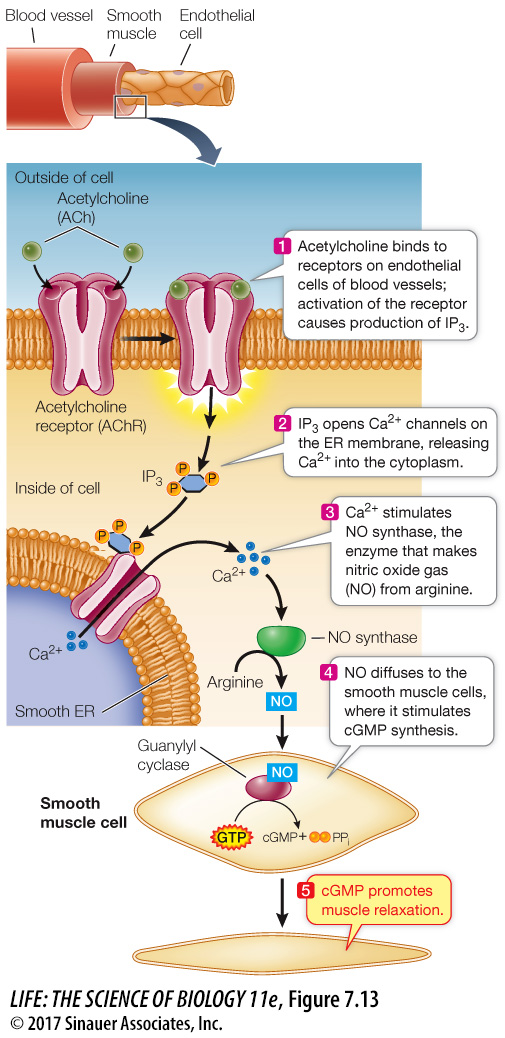
NITRIC OXIDE Most signaling molecules and second messengers are solutes that remain dissolved in either the aqueous or hydrophobic components of cells. It was a great surprise to find that a gas could also be active in signal transduction. Nitric oxide (NO) is a second messenger in the signal transduction pathway between the neurotransmitter acetylcholine (see Key Concept 7.2) and the relaxation of smooth muscles lining blood vessels, which allows more blood flow (Figure 7.13). In the body, NO is made from the amino acid arginine by the enzyme NO synthase. When the acetylcholine receptor on the surface of an endothelial cell is activated, IP3 is released from the membrane (via the pathway shown in Figure 7.13), causing a calcium channel in the ER membrane to open and a subsequent increase in cytosolic Ca2+. The Ca2+ then activates NO synthase to produce NO. NO is chemically very unstable, readily reacting with oxygen gas as well as other small molecules. Although NO diffuses readily, it does not get far. Conveniently, the endothelial cells are close to the underlying smooth muscle cells, where NO activates an enzyme called guanylyl cyclase (a close relative of adenylyl cyclase). This enzyme catalyzes the formation of cyclic GMP (cGMP): yet another second messenger that contributes to the relaxation of muscle cells.
The discovery of NO as a participant in signal transduction explained the action of nitroglycerin, a drug that has been used for more than a century to treat angina, the chest pain caused by insufficient blood flow to the heart. Nitroglycerin releases NO, which results in relaxation of the blood vessels and increased blood flow. The drug sildenafil (Viagra) was developed to treat angina via the NO signal transduction pathway but was only modestly useful for that purpose. However, men taking it reported more pronounced penile erections. During sexual stimulation, NO acts as a signal, causing an increase in cGMP and a subsequent relaxation of the smooth muscles surrounding the arteries in the corpus cavernosum of the penis. As a result of this signal, the penis fills with blood, producing an erection. Sildenafil acts by inhibiting an enzyme (a phosphodiesterase) that breaks down cGMP—
141