Receptors that recognize chemical signals have specific binding sites
134
A ligand is a specific chemical signal molecule that fits into a three-
The sensitivity of a cell to a signal is determined in part by the affinity of the cell’s receptors for the signal ligand—
R + L ⇌ RL (7.1)
For most ligand–
As with any reversible chemical reaction, the binding and dissociation processes each have a rate constant, here designated k1 and k2:

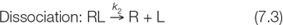
A rate constant relates the rate of a reaction to the concentration(s) of the reactant(s):
Rate of binding = k1[R][L] (7.4)
Rate of dissociation = k2[RL] (7.5)
where “[ ]” indicates the concentration of the substance inside the brackets. Binding of a receptor to a ligand is reversible, and when equilibrium is reached the rate of binding equals the rate of dissociation:
k1[R][L] = k2[RL] (7.6)
If this is rearranged, we get:

KD, the dissociation constant, is a measure of the affinity of the receptor for its ligand. The lower the KD, the higher the affinity of the ligand for its receptor. Some receptors have very low KD values, which allows them to bind their ligands at very low ligand concentrations; other receptors have higher KD values and need more ligand to be present to set off their signal transduction pathways.
An entire field of biology and medicine—
Question
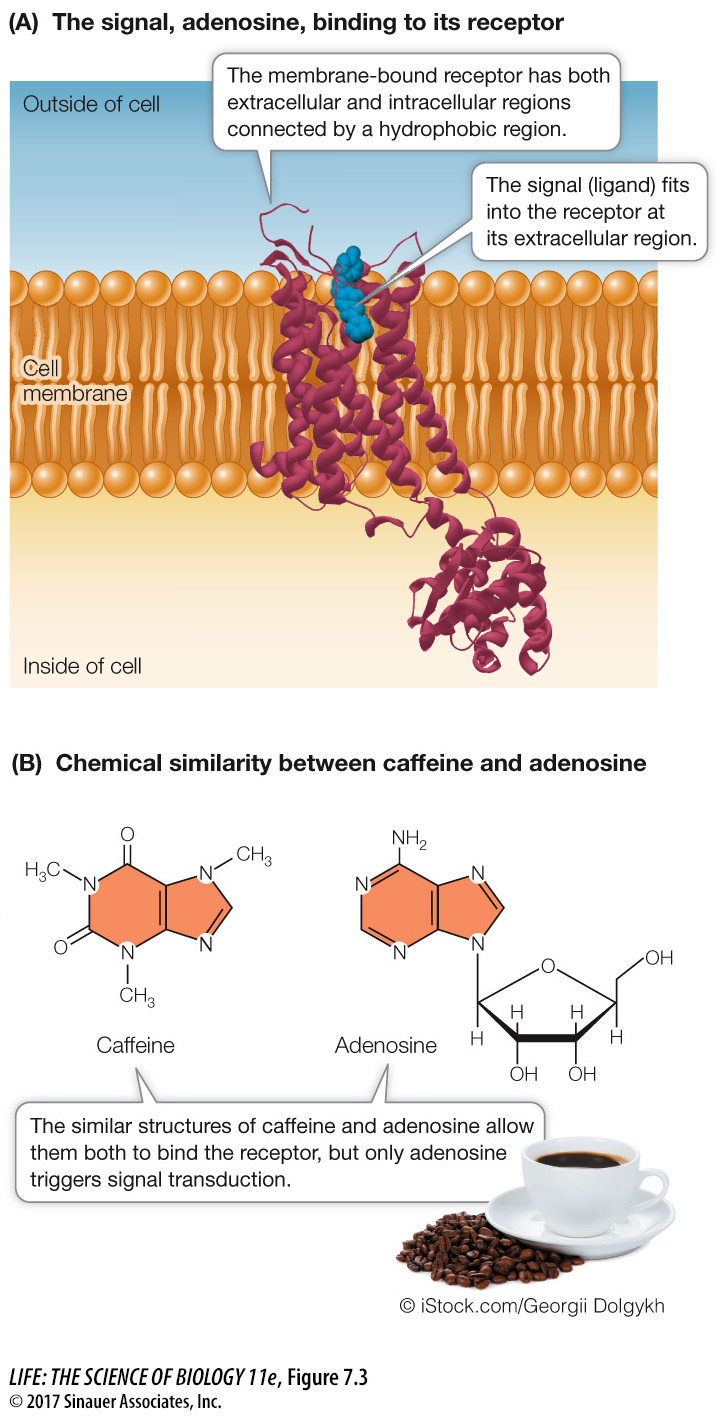
Q: Is the binding of adenosine and caffeine covalent or noncovalent? Explain your answer.
As with the binding of enzyme to substrate, the binding of caffeine and adenosine is noncovalent. Both substances bind to a specific site on the receptor by their shape and by interactions, including hydrophobic interactions (the rings).
What happens when a ligand binds to a receptor? When we discussed small molecules binding to proteins in Key Concept 3.2, we described how proteins often change shape when bound. This is exactly what happens to receptors. The change in the receptor’s shape may expose a previously hidden group of amino acids on the protein that participate in a biochemical activity. This activity may be the binding of another molecule, such as another protein (e.g., a G protein, discussed below), or a substrate for an enzyme.
Instead of the ligand, other chemicals that resemble it can bind to the receptor. Agonists are chemicals that set a receptor into signal transduction mode just as the ligand does. In contrast, inhibitors, or antagonists, bind to the receptor and “freeze” it in place, preventing the real ligand from binding, but do not set off signal transduction. Agonists and antagonists can be natural, or we can make them in the lab once we know the details of receptor–
135
Many substances that alter human behavior bind to specific receptors in the brain and prevent the binding of the receptors’ specific ligands. One example is caffeine, which is probably the world’s most widely consumed stimulant. In the brain, the nucleoside adenosine acts as a ligand that binds to a receptor on nerve cells, initiating a signal transduction pathway that reduces brain activity, especially feelings of active wakefulness. Because caffeine has a molecular structure similar to that of adenosine, it also binds to the adenosine receptor (Figure 7.3B). But in this case binding does not initiate a signal transduction pathway. Rather, it “ties up” the receptor, preventing adenosine binding and thereby allowing continued nerve cell activity and an active feeling.