Receptors can be classified by location and function
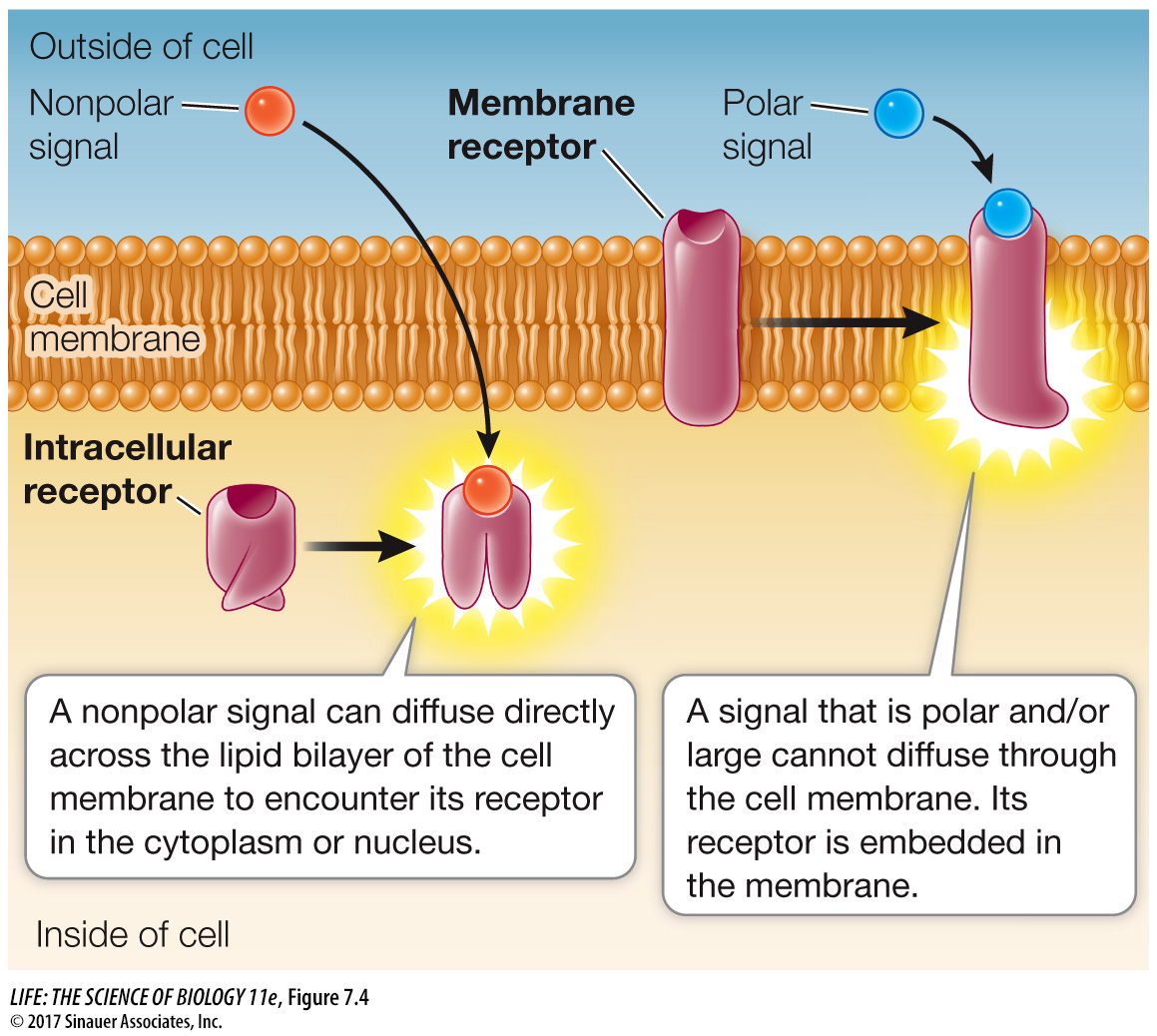
There are many kinds of chemical signals. Some ligands are hydrophobic (nonpolar) and can diffuse through membranes, whereas others cannot. Physical signals such as light also vary in their ability to penetrate cells and tissues. Correspondingly, a receptor can be classified by its location in the cell, which largely depends on the nature of its signal (Figure 7.4):
Membrane receptors: Large or polar ligands cannot cross the lipid bilayer. Insulin, for example, is a protein hormone that cannot diffuse through the cell membrane; instead, it binds to a transmembrane receptor with an extracellular binding domain.
Intracellular receptors: Small or nonpolar ligands can diffuse across the nonpolar phospholipid bilayer of the cell membrane and enter the cell. The hormone estrogen, for example, is a lipid-
soluble steroid (see in- text art p. 61) that can diffuse across the cell membrane; it binds to a receptor inside the cell. Light of certain wavelengths can penetrate the cells in a plant leaf quite easily, and many types of light receptors in plants are also intracellular.
In complex eukaryotes such as mammals and higher plants, there are three well-
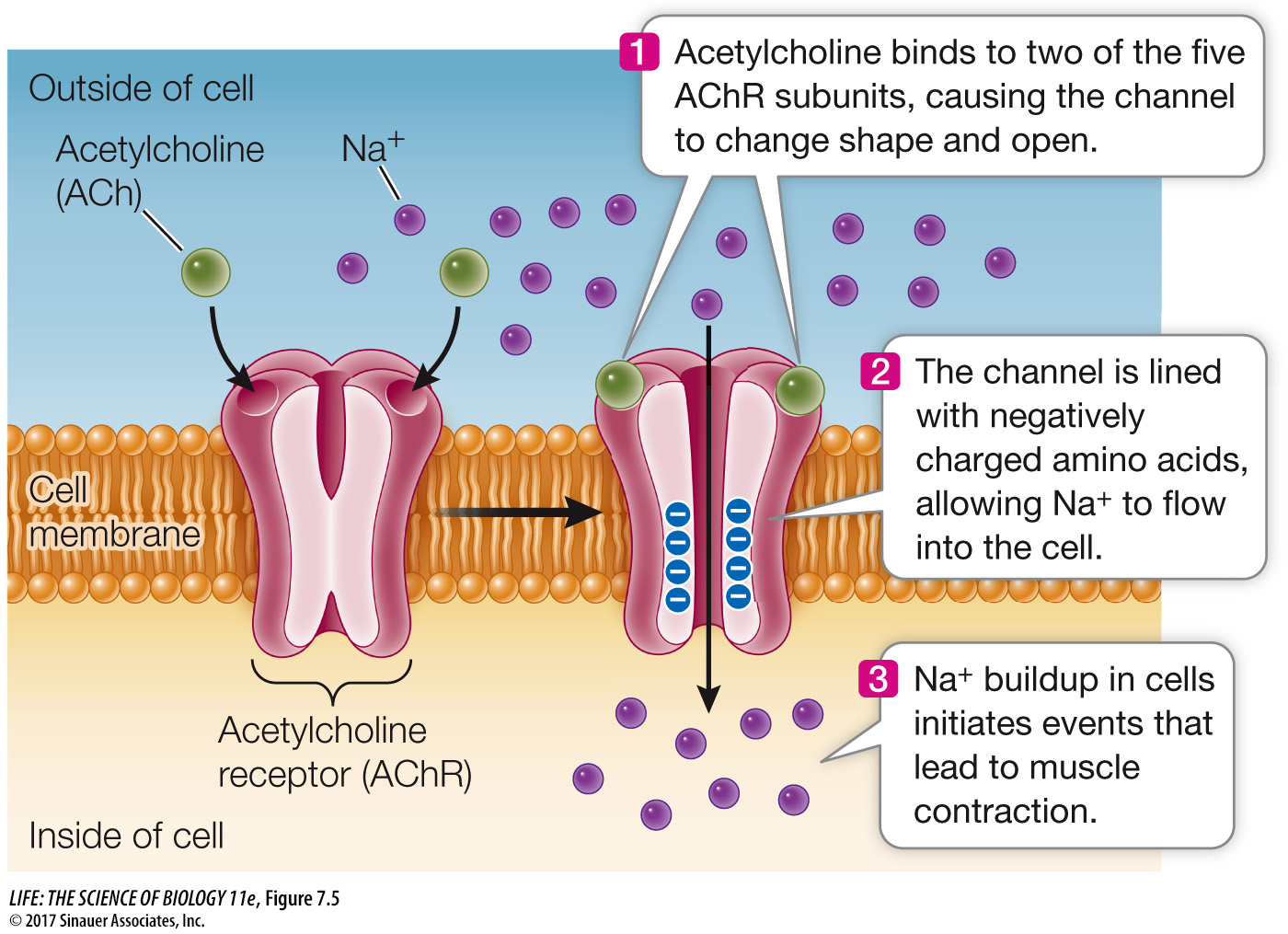
ION CHANNELS As you saw in Key Concept 6.3, the cell membranes of many types of cells have gated ion channels that allow ions such as Na+, K+, Ca2+, or Cl– to enter or leave the cell. The gate-
*connect the concepts Ion channels are key to the functioning of the nervous system. An example is the connection between nerve and muscle described in Key Concepts 44.3 and 47.1.
The acetylcholine receptor, located in the cell membrane of skeletal muscle cells, is an example of an ion channel. This protein is a sodium channel that binds the ligand acetylcholine, which is a neurotransmitter—
136
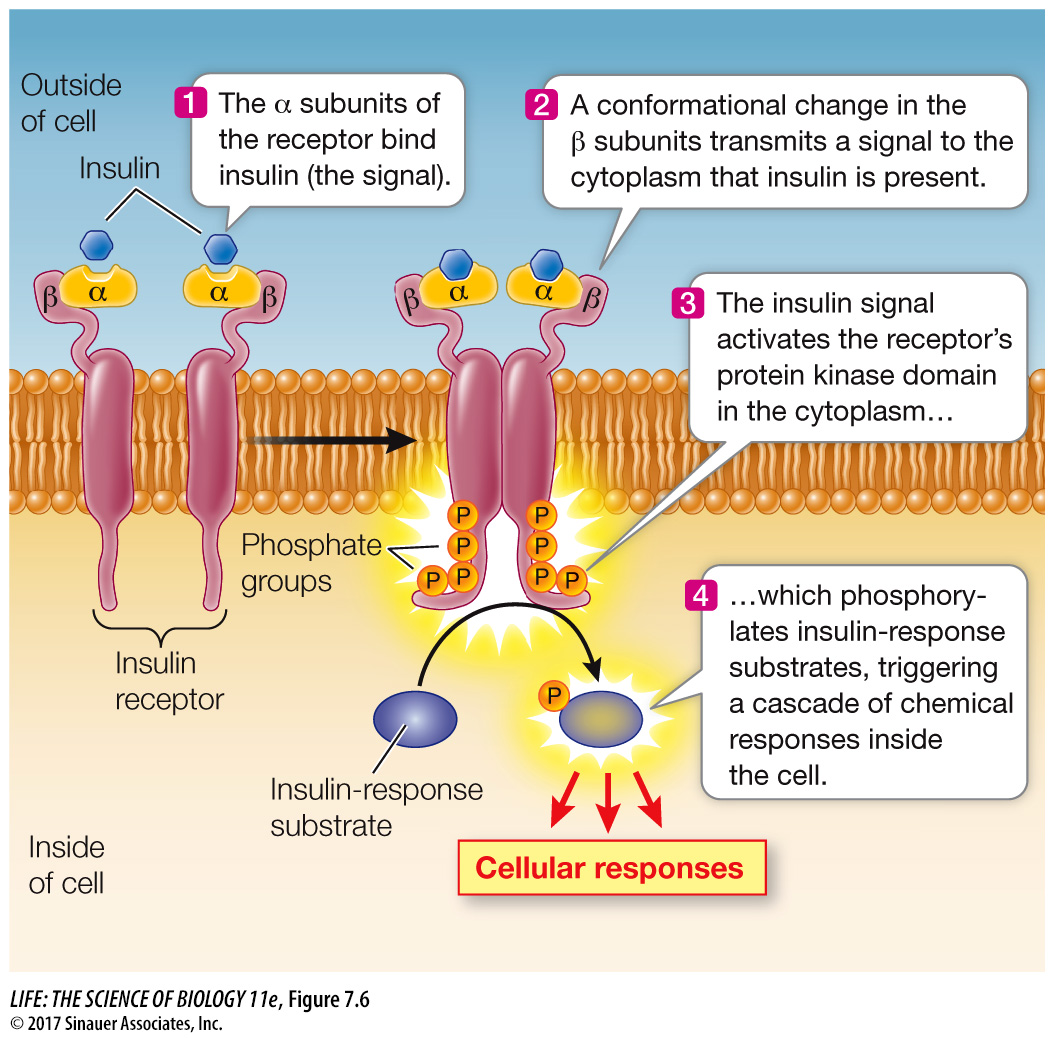
PROTEIN KINASE RECEPTORS Some eukaryotic receptor proteins, called protein kinases, catalyze the phosphorylation (adding phosphate) of themselves or other proteins, thus changing their shapes and therefore their functions.
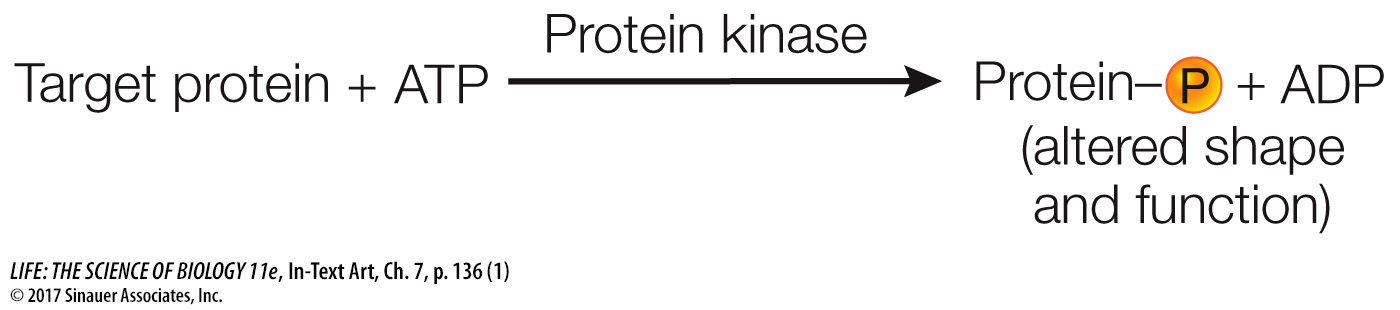
Phosphorylation is a reaction that is especially important in biology. Of the estimated 21,000 genes that code for proteins in humans, more than 500 of them encode protein kinases. When you think of all the functions that make up a person, this is indeed an impressive number. The three amino acids that are phosphorylated are therefore worth knowing:
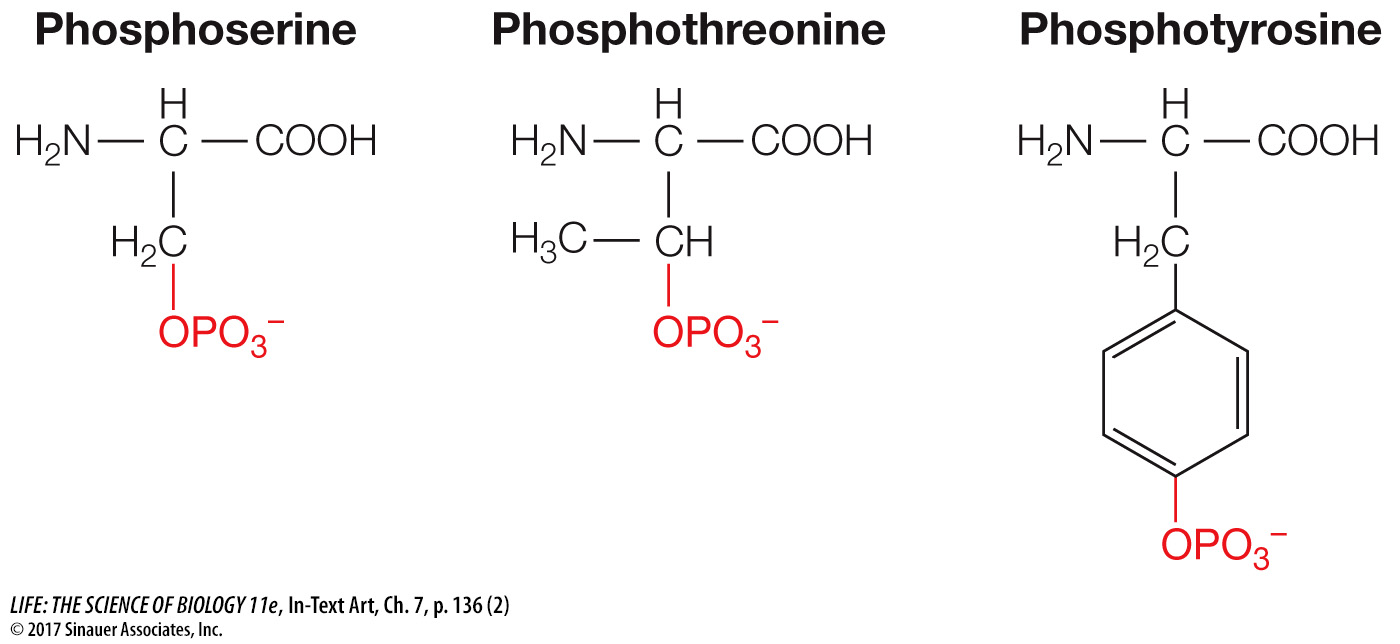
The receptor for insulin is an example of a protein kinase receptor. Insulin is a protein hormone made by the pancreas. Its receptor has two copies each of two different polypeptide subunits called α and β (Figure 7.6). When insulin binds to the receptor, the receptor becomes activated and is able to phosphorylate itself and certain cytoplasmic proteins that are appropriately called insulin-
Animation 7.1 A Signal Transduction Pathway
www.life11e.com/
G PROTEIN-
The seven-
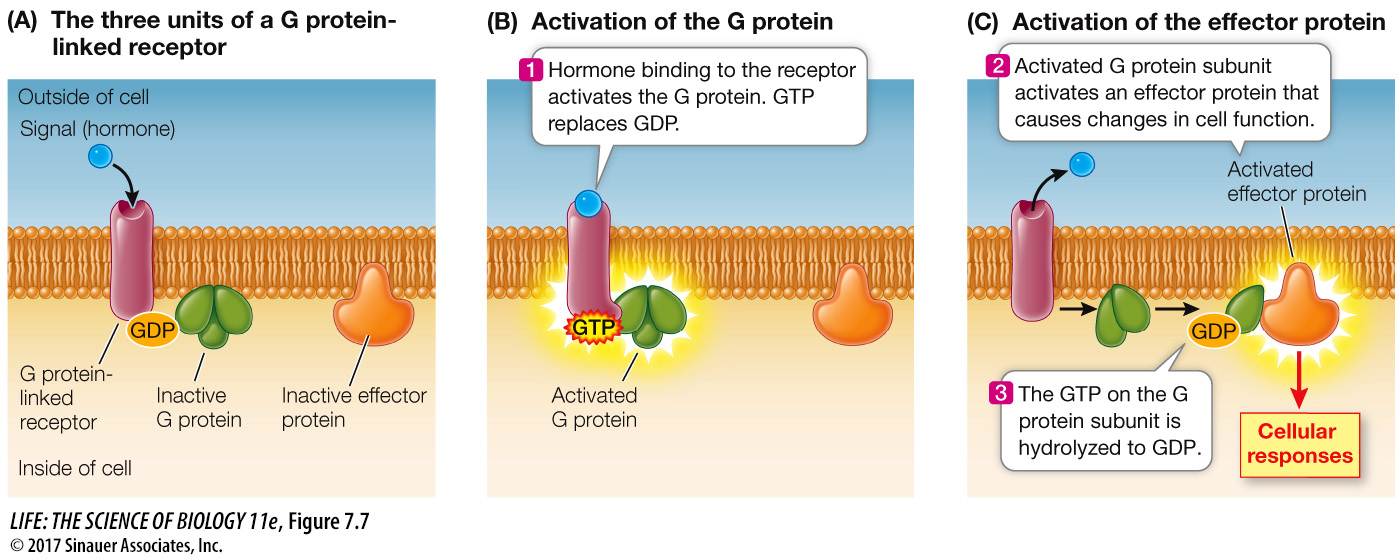
Many G proteins have three polypeptide subunits and can bind three different types of molecules (Figure 7.7A):
137
The receptor
GDP and GTP (guanosine diphosphate and triphosphate, respectively; these are nucleoside phosphates like ADP and ATP)
An effector protein (see next paragraph)
When the G protein binds to an activated receptor protein, GDP is exchanged for GTP (Figure 7.7B). At the same time, the ligand is usually released from the extracellular side of the receptor. GTP binding causes a conformational change in the G protein. The GTP-
After activation of the effector protein, the GTP bound to the G protein is hydrolyzed to GDP. The now inactive G protein subunit separates from the effector protein and diffuses in the membrane to collide with and bind to the other two G protein subunits. When the three components of the G protein are reassembled, the protein is capable of binding again to an activated receptor. After binding, the activated receptor exchanges the GDP on the G protein for a GTP, and the cycle begins again.