ATP hydrolysis releases energy
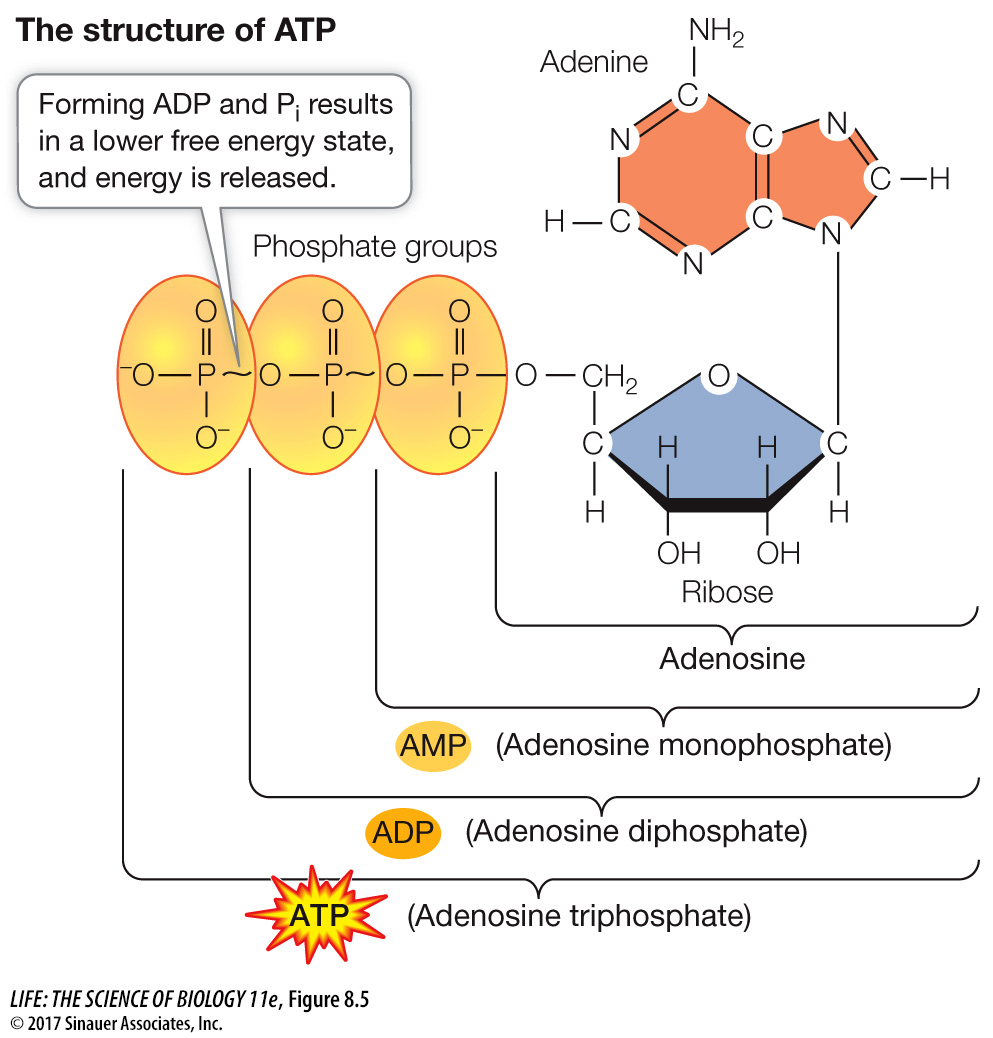
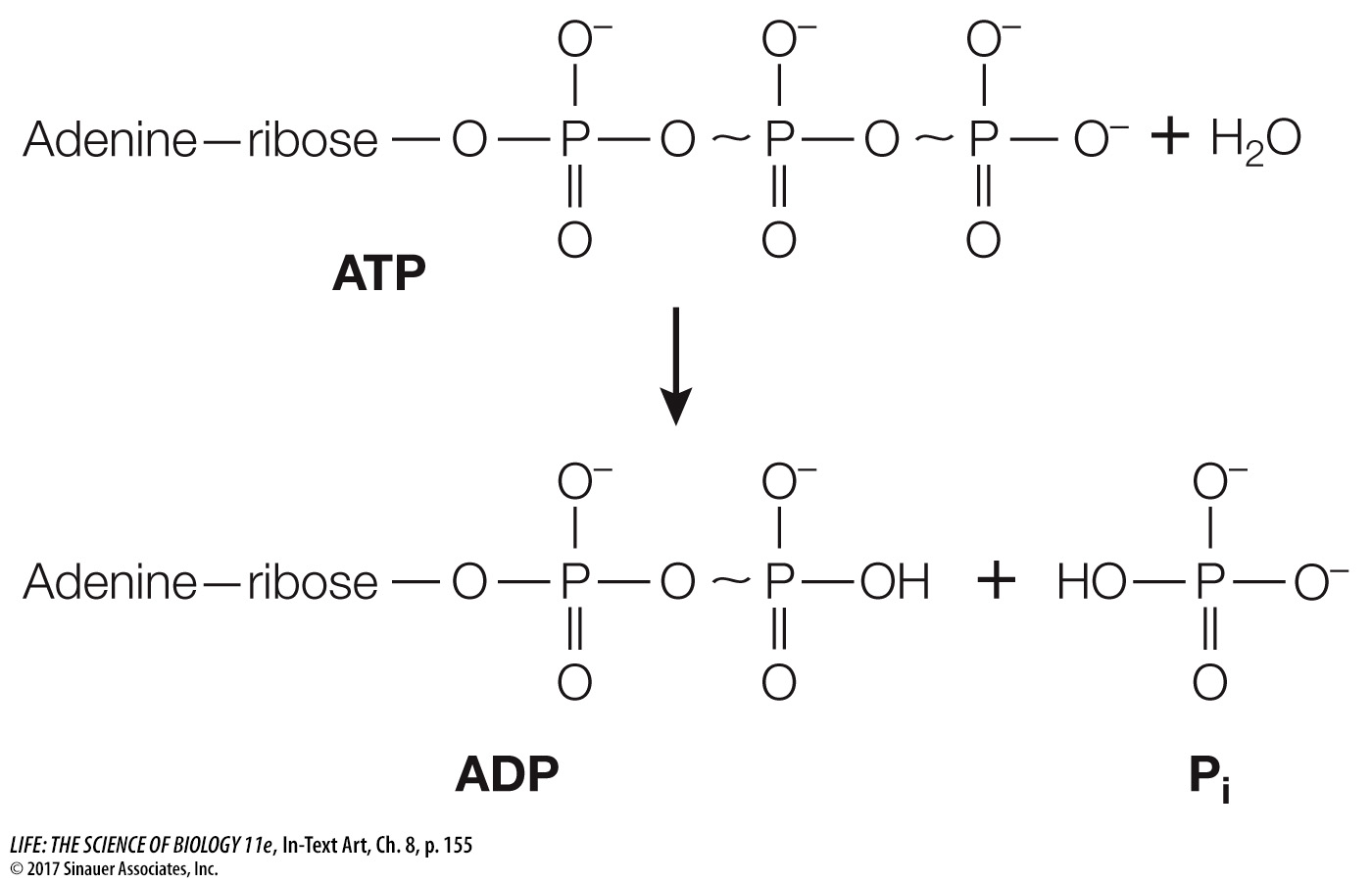
An ATP molecule consists of the nitrogenous base adenine bonded to ribose (a sugar), which is attached to a sequence of three phosphate groups (Figure 8.5A). The hydrolysis of a molecule of ATP yields free energy, as well as ADP and an inorganic phosphate ion (Pi). Thus:
ATP + H2O → ADP + Pi + free energy
Activity 8.1 ATP and Coupled Reactions
www.life11e.com/
The important property of this reaction is that it is exergonic, releasing free energy. Under standard laboratory conditions, the change in free energy (ΔG) for this reaction is about –7.3 kcal/mol (–30 kJ/mol). However, under cellular conditions ΔG can be as much as –14 kcal/mol. We give both values here because you will encounter them both and you should be aware of their origins. Both are correct, but in different conditions.
A molecule of ATP can be hydrolyzed either to ADP and Pi, or to adenosine monophosphate (AMP) and a pyrophosphate ion (P2O74–
Because phosphate groups are negatively charged and so repel each other, it takes energy to get two phosphates near enough to each other to make the covalent bond that links them together. Some of this energy is stored as potential energy in the P~O bonds between the phosphates in ATP (the wavy line indicates a high-
energy bond). The free energy of this P~O bond (called a phosphoric acid anhydride bond) is much higher than the energy of the O—
H bond that forms as a result of hydrolysis. So it is the lower free energy state of the system that releases energy.
156
Cells use the energy released by ATP hydrolysis to fuel endergonic reactions (such as the biosynthesis of complex molecules), for active transport, for movement, and even for the production of light (bioluminescence). Another interesting example of the use of ATP involves converting its chemical energy into light energy.
Media Clip 8.1 Bioluminescence in the Deep Sea
www.life11e.com/