To speed up a reaction, an energy barrier must be overcome
An exergonic reaction may release free energy, but without a catalyst it will take place very slowly because there is an energy barrier between reactants and products. Think about the hydrolysis of sucrose, which we described in Key Concept 8.1:
Sucrose + H2O → glucose + fructose
In your body, this reaction is part of digestion. Even if water is abundant, the sucrose molecule will only very rarely bind the H atom and the —OH group of water at the appropriate locations to break the covalent bond between glucose and fructose unless there is an input of energy to initiate the reaction. Such an input of energy will place the sucrose into a reactive mode called the transition state. The energy input required for sucrose to reach this state is called the activation energy (Ea). Here’s a more familiar example that can show you the ideas of activation energy and transition state:
Fireworks + O2 → CO2 + H2O + energy (heat and light)
Fireworks don’t start exploding until you give them a spark: that’s the activation energy. It excites the molecules in the fireworks so they will react with oxygen in the air. Once the transition state is reached, the explosive reaction occurs.
158
Generally, exergonic reactions proceed only after the reactants are pushed over the energy barrier by some added energy. The energy barrier thus represents the amount of energy needed to start the reaction, the activation energy (Figure 8.8A. The ball has a lot of potential energy at the top of the hill. However, if it is stuck in a small depression, it will not roll down the hill, even though that action is exergonic. To start the ball rolling, a small amount of energy (activation energy) is needed to push it out of the depression (Focus: Key Figure 8.8B). In a chemical reaction, the activation energy is the energy needed to change the reactants into unstable molecular forms called transition-
focus: key figure
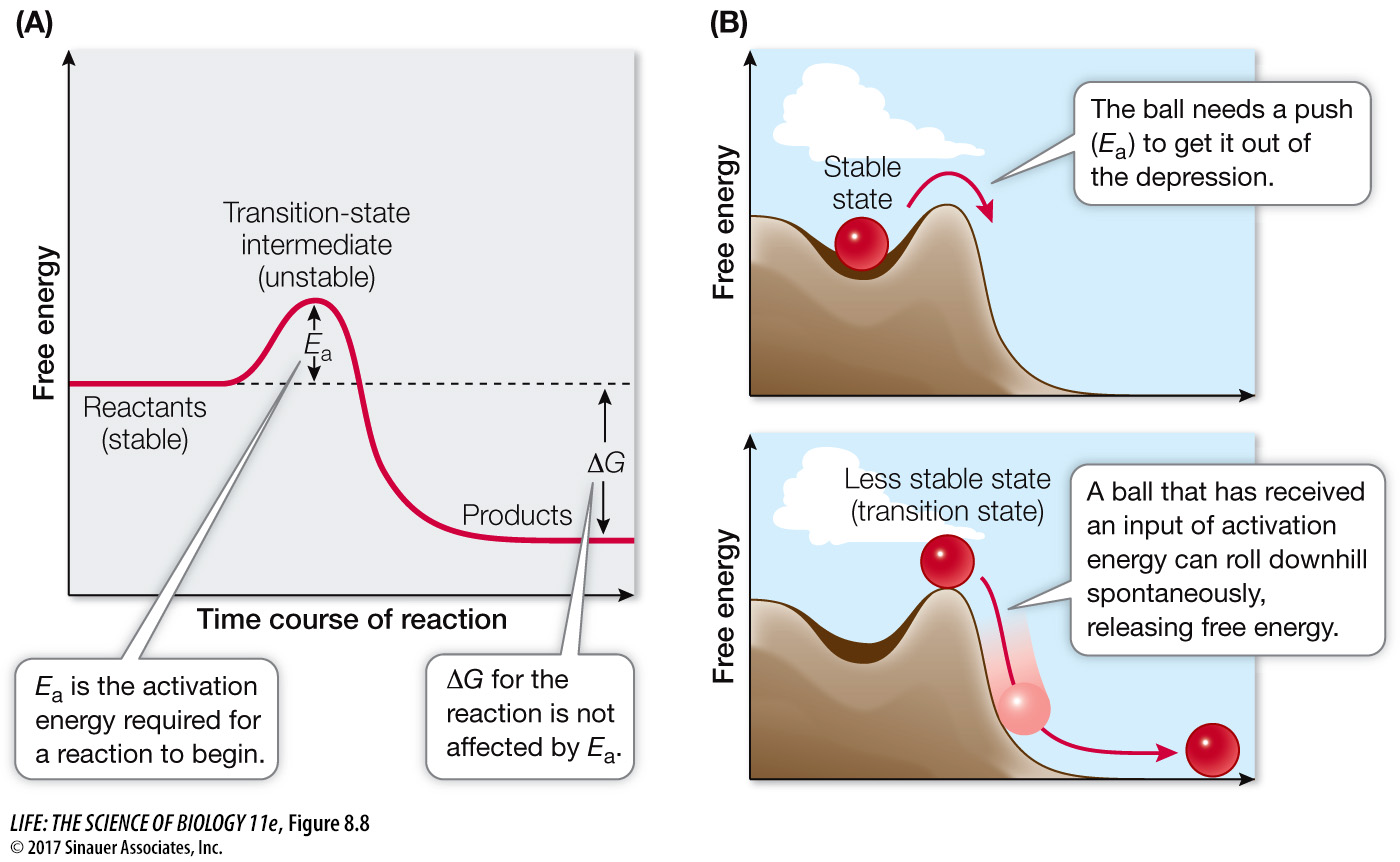
Question
Q: Would activation energy be required for an endergonic reaction?
Yes. In this case, activation energy is needed to make the substrate(s) able to take up energy and then the reaction proceeds.
Transition-
Where does activation energy come from? If reactants are at the human body temperature (37°C), they are moving around. A few are moving fast enough that their kinetic energy can overcome the energy barrier, enter the transition state, and react. So the reaction takes place—
However, adding heat to increase the average kinetic energy of the molecules so they can react would not work in living systems. Such a nonspecific approach would accelerate all chemical reactions, including destructive ones such as the denaturation of proteins (see Chapter 3). A more effective way to speed up a reaction in a living system is to lower the energy barrier by bringing the reactants close together. In living cells, enzymes accomplish this task.