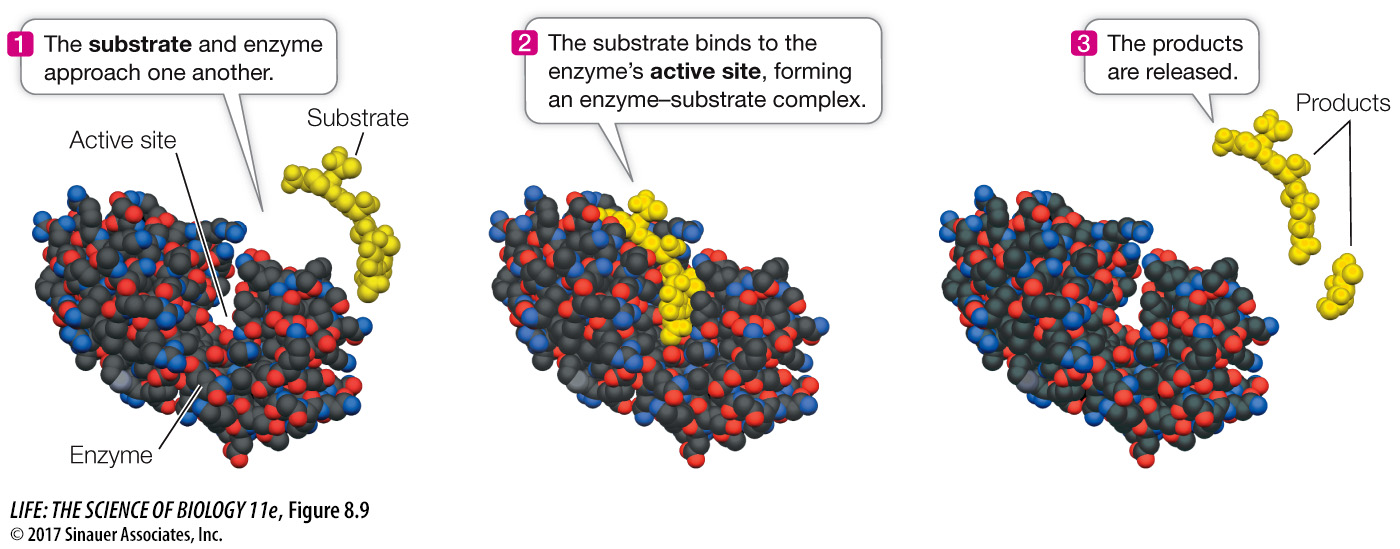
Enzymes bind specific reactants at their active sites
Catalysts increase the rates of chemical reactions. Most nonbiological catalysts are nonspecific. For example, powdered platinum catalyzes virtually any reaction in which molecular hydrogen (H2) is a reactant. In contrast, most biological catalysts are highly specific. An *enzyme usually recognizes and binds to only one or a few closely related reactants, yielding specific products.
*connect the concepts To understand enzymes and their activity, you must first understand the structures and chemical properties of proteins. See Key Concept 3.2.
In an enzyme-
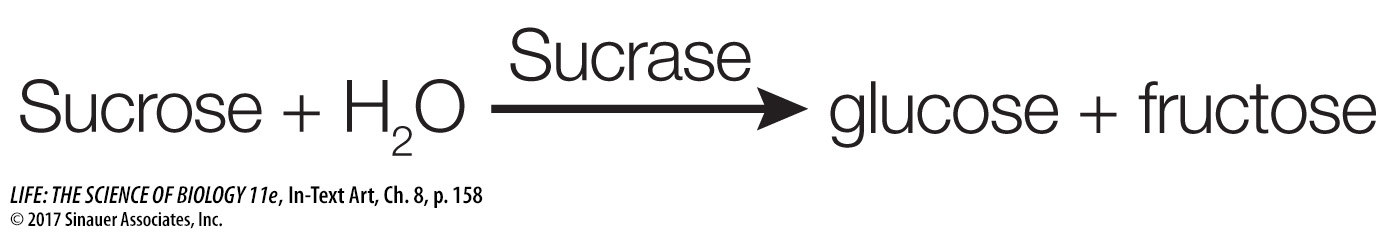
The names of enzymes often reflect their functions and end with the suffix “ase.” For example, the enzyme sucrase catalyzes the hydrolysis of sucrose, which we have referred to above:
159
Thousands of enzymes have been identified, each catalyzing the transformation of its particular substrate(s). In general, enzymes fall into six categories:
Oxidoreductases transfer electrons between molecules, especially in energy metabolism. An example of an enzyme that catalyzes this reaction is oxidoreductase:
A–
+ B → A + B– You will see other examples in the next two chapters.
Transferases transfer groups of atoms (functional groups) (see Figure 3.1) between molecules. The basic reaction can be shown as:
AX + B → A + BX
where A = the donor, B = the acceptor, and X = the functional group. For example, aminotransferases transfer –NH2 groups between molecules, linking carbohydrates and amino acids.
Hydrolases add water to covalent bonds to break down molecules. An example (shown above) is sucrase, which hydrolyzes sucrose to its constituent monosaccharides.
Lyases catalyze the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure. For example, a lyase catalyzes the cleavage of the phosphorus–
oxygen bond of ATP: ATP → cAMP + PPi
Isomerases move functional groups from one place to another within a molecule, forming an isomer with the same atoms, differently bonded. For example, glucose and fructose are isomers of C6H12O6 (see Figure 3.17).
Ligases join two molecules together. You will see examples of ligases when you learn about DNA replication in Chapter 13.
A special note on language: When biologists refer to an enzyme, they usually use a verb for its action. For example, as noted above (#6), we used the words “ligases join. . ..” It is important for you to realize that this wording is shorthand for what really happens: ligases catalyze the joining. Enzymes don’t “do” the reaction; they catalyze it.
When a substrate binds to the active site of an enzyme, the result is called an enzyme–
E + S → ES → E + P
where E is the enzyme, S is the substrate, P is the product, and ES is the enzyme–
*connect the concepts Binding of a substrate to an enzyme involves not just a “fit” but also noncovalent interactions between the enzyme and substrate. See Key Concept 3.2 for an outline of noncovalent interactions.
The relationship between enzyme and substrate may seem familiar; it is analogous to the binding of a receptor and ligand that we described in Chapter 7. In that instance, we described the affinity of a receptor for its ligand with the dissociation constant (KD). The lower the KD, the tighter the binding. For enzymes and their substrates, KD values are often in the range 10–5 to 10–6 M, which favors the formation of the ES. In practical terms, the KD values of the ES are such that the binding between enzyme and substrate is somewhat reversible; the substrate can be released before the reaction. However, many enzyme-