The substrate concentration affects the reaction rate
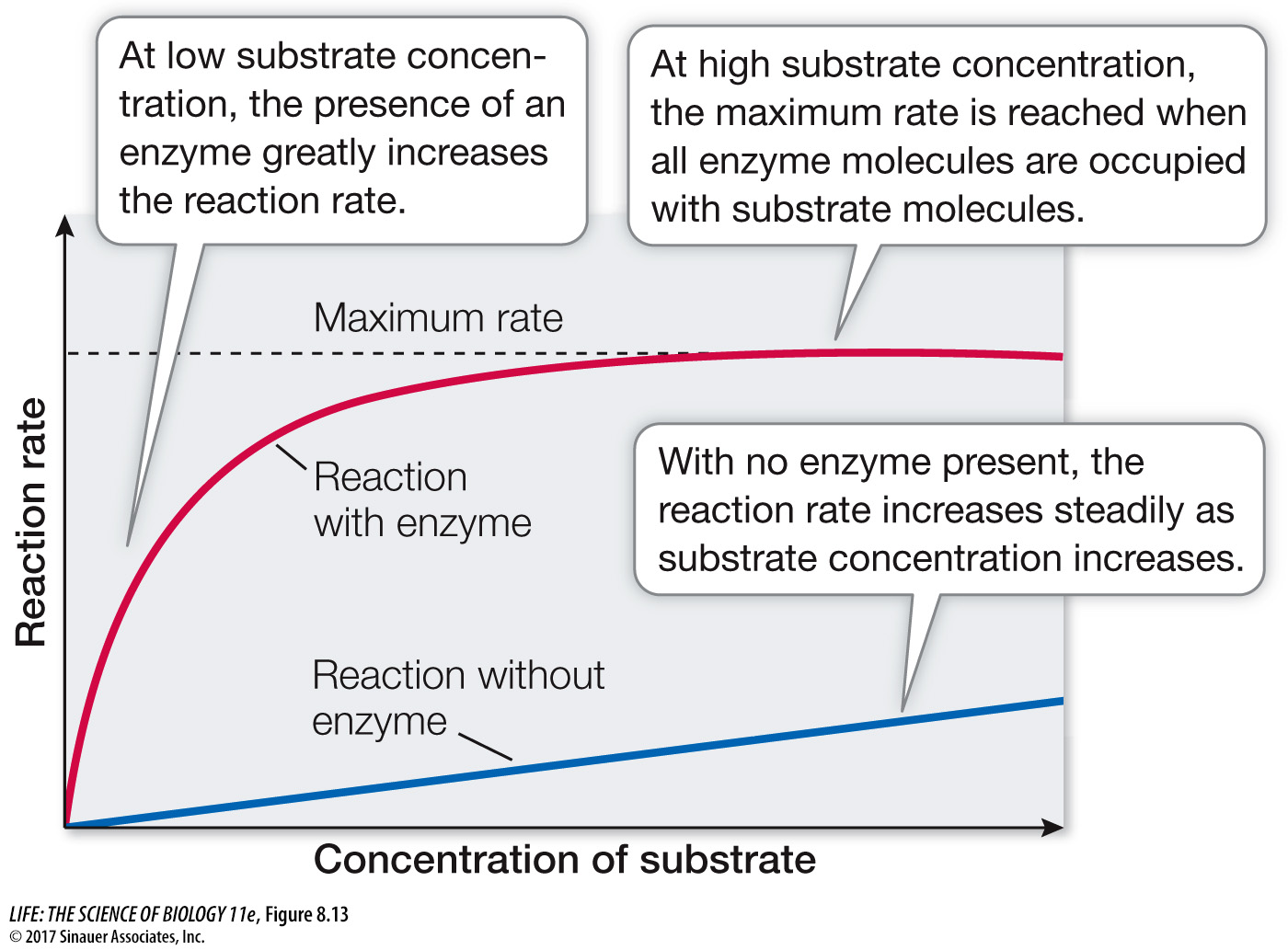
For a reaction of the type A → B, the rate of the uncatalyzed reaction is directly proportional to the concentration of A. The higher the concentration of substrate, the faster the rate of the reaction. The appropriate enzyme not only speeds up the reaction; it also changes the shape of a plot of rate versus substrate concentration (Figure 8.13). For a given concentration of enzyme, the rate of the enzyme-
163
The concentration of an enzyme is usually much lower than that of its substrate (e.g., in cells expressing the enzyme sucrase, there are far more molecules of sucrose than of the enzyme). As more and more substrate molecules bind to the available enzyme molecules, there is a saturation phenomenon like the one that occurs in facilitated diffusion (see Figure 6.12). When all the enzyme molecules are bound with substrate molecules, the enzyme is working as fast as it can—
The maximum rate of a catalyzed reaction can be used to measure how efficient the enzyme is. The turnover number is the maximum number of substrate molecules that one enzyme molecule can convert to product per unit of time. This number ranges from 1 molecule every 2 seconds for lysozyme to an amazing 40 million molecules per second for the liver enzyme catalase.