recap
8.4 recap
Enzymes orient their substrates to bring together specific atoms so that bonds can form. An enzyme can participate in the reaction it catalyzes by temporarily changing shape or destabilizing the enzyme–
learning outcomes
You should be able to:
Give examples of mechanisms for enzyme catalysis.
Describe coenzymes and cofactors and how they function.
Predict changes in the rate of an enzyme-
catalyzed reaction as factors in the reaction are varied.
Question 1
The chemical composition of an enzyme before and after a catalyzed reaction is the same, but during the reaction an enzyme can change. How and why does this happen?
As part of the catalysis, an enzyme can add or remove H+ in a substrate, which may facilitate reaction with the substrate. The course of these H+ reactions involves acidic or basic R groups on amino acids at the active site. Metal ions of enzymes may gain or lose electrons during the reaction, donating them temporarily to the substrate.
Question 2
Compare enzymes and coenzymes with regard to chemical structure and function.
Coenzymes are small and nonprotein; enzymes are large proteins or RNA. Coenzymes do not catalyze reactions; enzymes are catalysts. Coenzymes add or remove chemical groups (e.g., H+) and are permanently changed as a result of the reaction; enzymes are not permanently changed by participating in a reaction.
Question 3
Plot reaction rate versus substrate concentration, and explain the shape of the curve.
At the beginning, the reaction rate will increase linearly with added enzyme since the enzyme will be saturated with substrate even after many additions. Thus the reaction rate will increase proportionally with the amount of enzyme added, but only until the enzyme is no longer saturated with substrate. At this point the enzyme will be present in excess with respect to substrate, and additional enzyme will have no additional effect. The curve will level off to a constant maximum rate when this point is reached. 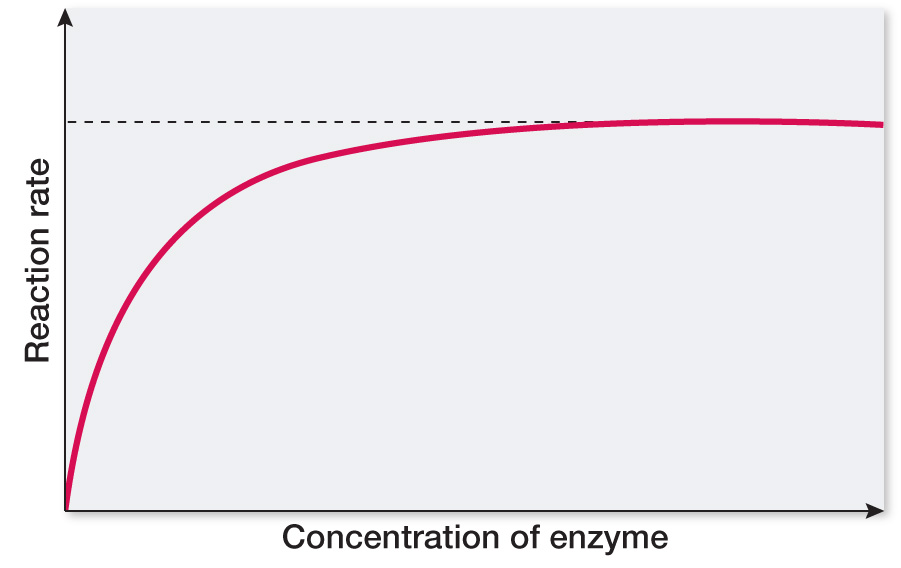
You’ve seen in this section how individual enzymes work on their substrates. However, enzymes inside organisms don’t operate in isolation—