Allosteric enzymes are controlled via changes in shape
The change in enzyme shape that is due to noncompetitive inhibitor binding is an example of allostery (allo, “different,” + stereos, “shape”). Allosteric regulation occurs when an effector molecule binds to a site other than the active site of an enzyme, inducing the enzyme to change its shape. The change in shape alters the affinity of the active site for the substrate, and so the rate of the reaction is changed.
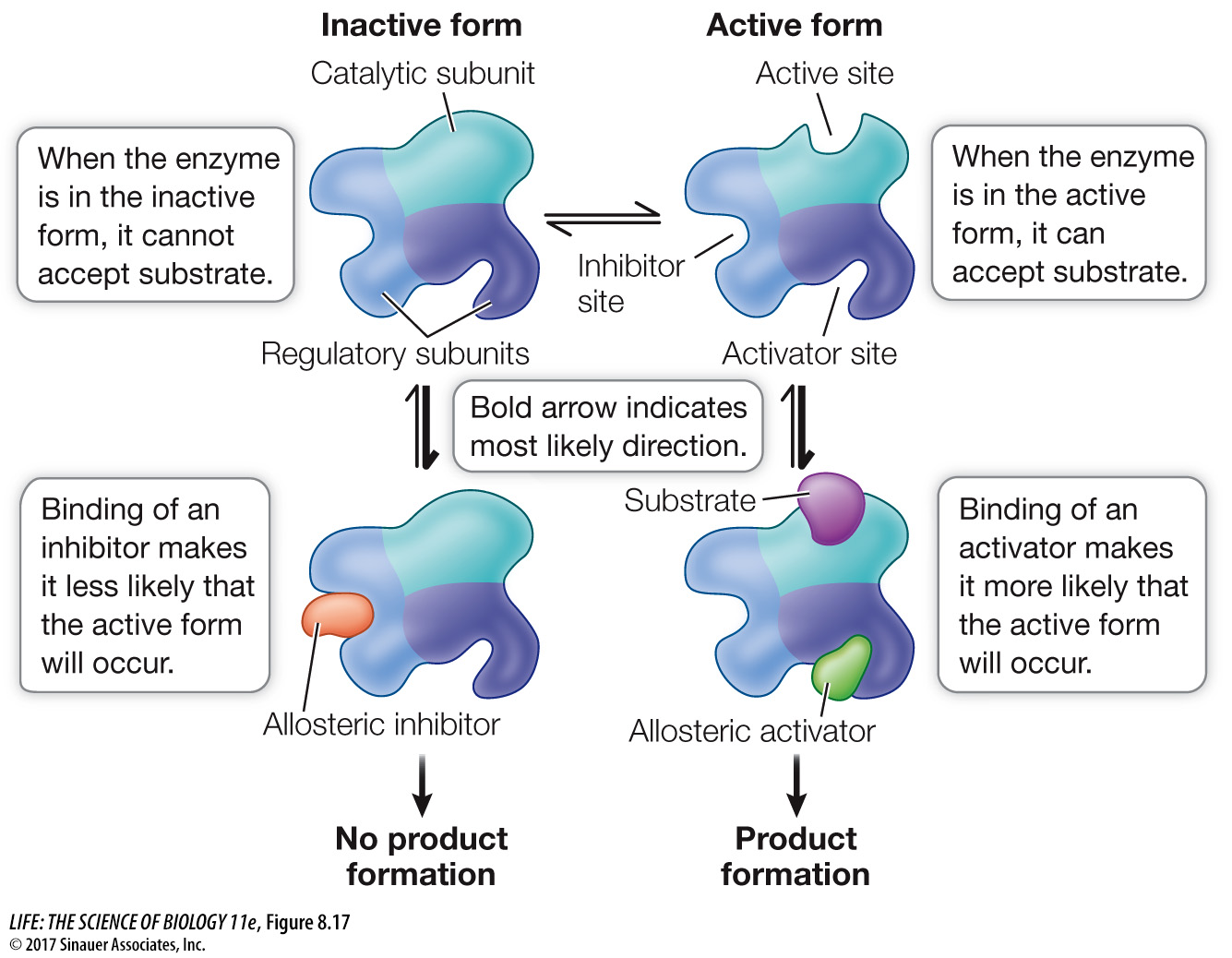
Often, an enzyme will exist in the cell in more than one possible shape (Figure 8.17):
Animation 8.2 Allosteric Regulation of Enzymes
www.life11e.com/
The active form of the enzyme has the proper shape for substrate binding.
The inactive form of the enzyme has a shape that cannot bind the substrate.
Other molecules, collectively referred to as effectors, can influence which form the enzyme takes:
Binding of an inhibitor to a site other than the active site can stabilize the inactive form of the enzyme, making it less likely to convert to the active form.
The active form can be stabilized by the binding of an activator to another site on the enzyme.
167
Like substrate binding, the binding of inhibitors and activators to their regulatory sites (also called allosteric sites) is highly specific. Most (but not all) enzymes that are allosterically regulated are proteins with quaternary structure; that is, they are made up of multiple polypeptide subunits. The polypeptide that has the active site is called the catalytic subunit. The allosteric sites are often located on different polypeptides, called the regulatory subunits (see Figure 8.17).
Some enzymes have multiple subunits containing active sites, and the binding of substrate to one of the active sites causes allosteric effects. When substrate binds to one subunit, there is a slight change in protein structure that influences the adjacent subunit. The slight change to the second subunit makes its active site more likely to bind to the substrate. So the reaction speeds up as the sites become sequentially activated.
As a result, an allosteric enzyme with multiple active sites and a nonallosteric enzyme with a single active site differ greatly in their reaction rates when the substrate concentration is low. Graphs of reaction rates plotted against substrate concentrations show this relationship. For a nonallosteric enzyme, the plot is hyperbolic:
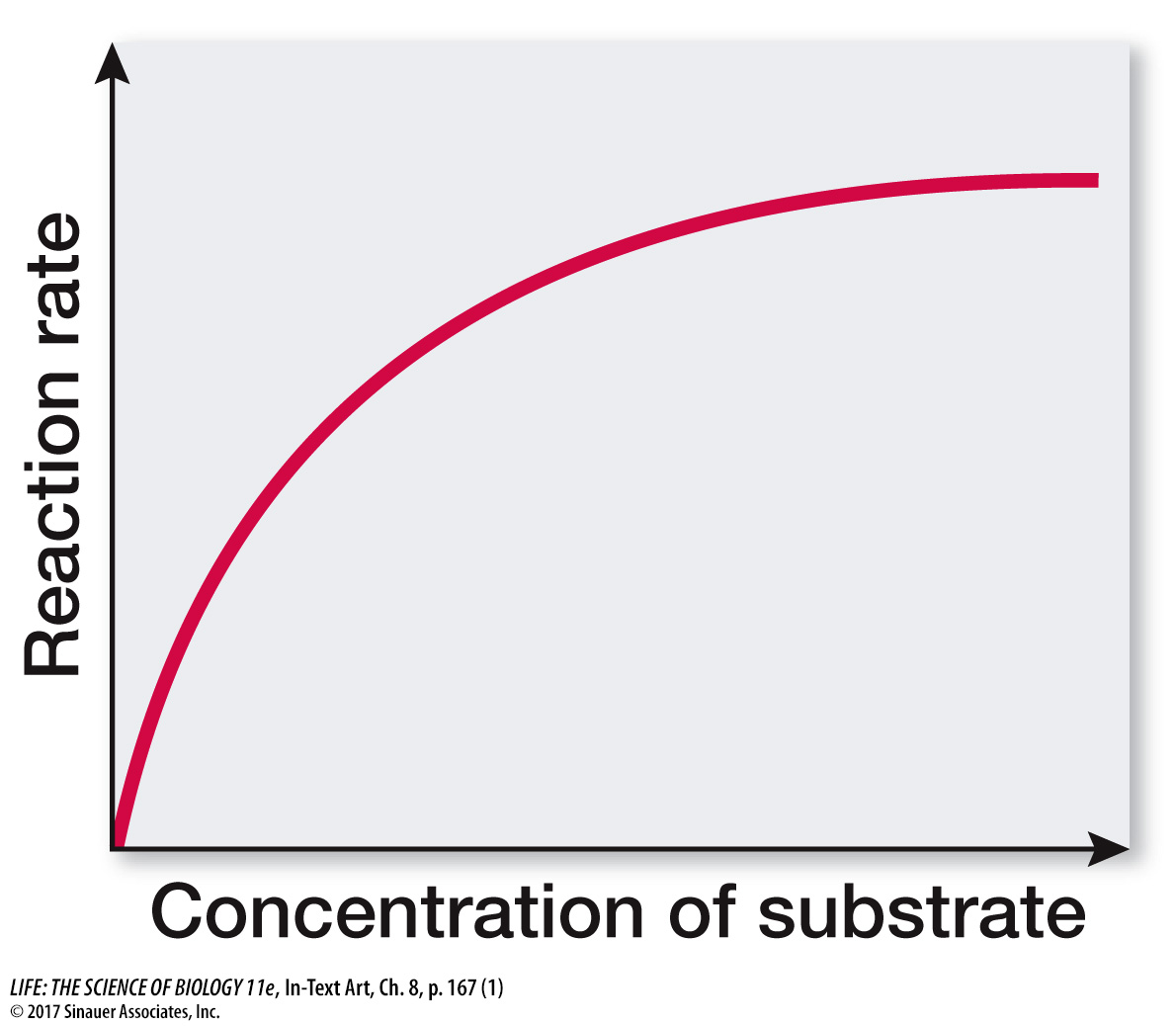
The reaction rate first increases sharply with increasing substrate concentration, then tapers off to a constant maximum rate as the supply of enzyme becomes saturated.
For a multisubunit allosteric enzyme, the graph looks different, having a sigmoid (S-
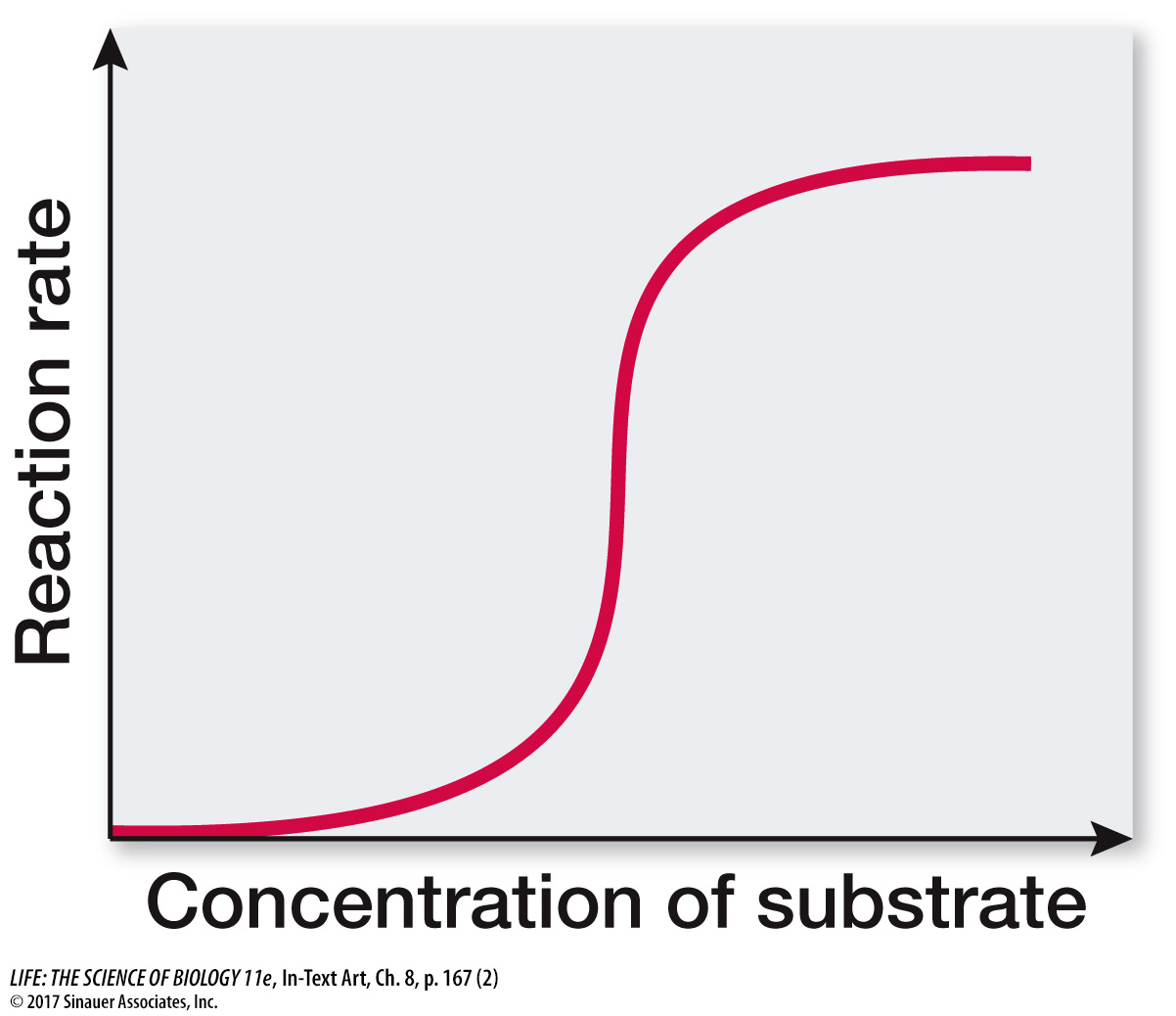
At low substrate concentrations, the reaction rate increases only gradually as substrate concentration increases. After the substrate binds to the first active site of the enzyme (the slowly increasing part of the curve), there is a change in the enzyme’s quaternary structure such that the other sites become more likely to bind substrate, so the reaction speeds up (the rapidly increasing part of the curve). Once all sites are saturated with substrate, the reaction rate reaches a plateau (the upper, flat part of the curve). Within a certain range, the reaction rate is extremely sensitive to relatively small changes in substrate concentration. In addition, allosteric enzymes are very sensitive to low concentrations of inhibitors. Because of this sensitivity, allosteric enzymes are important in regulating entire metabolic pathways.