Apply What You’ve Learned
171
Review
8.2 ATP releases usable energy for a cell when hydrolyzed to ADP and Pi.
8.2 Through coupling reactions, ATP drives endergonic reactions using energy derived from exergonic reactions.
8.3 Catalysts speed up the rate of a reaction but do not allow the occurrence of a reaction that would not otherwise take place.
8.4 An enzyme may undergo a change in shape called induced fit as the result of binding its substrate.
8.5 Environmental factors such as pH and temperature affect enzyme activity.
Original Papers: McElroy, W. D. 1947. The energy source for bioluminescence in an isolated system. Proceedings of the National Academy of Sciences USA 33: 342–
McElroy, W. D. and B. L. Strehler. 1954. Bioluminescence. Bacteriological Reviews 18: 177–
Thompson, J. F. et al. 1997. Mutation of a protease-
Many people enjoy watching and catching fireflies on warm summer nights, but the attraction isn’t mutual; a firefly’s primary goal is to find a mate. Male and female fireflies use specific patterns of light flashes to attract one another. The flashes are emitted from the firefly lantern, an organ located in the firefly abdomen.
Light is a form of energy, which means that fireflies must divert some of their energy to produce light flashes. Researchers used a biochemical approach to learn how this is done. When the lanterns of fireflies are ground up and extracted with water, the extract briefly produces an intense light, which then fades away. If ATP is added to the extract, light is again emitted, and again, it fades. The graph below shows the duration of light emission as a function of the amount of ATP added. When anaerobic conditions were used, no light was observed.
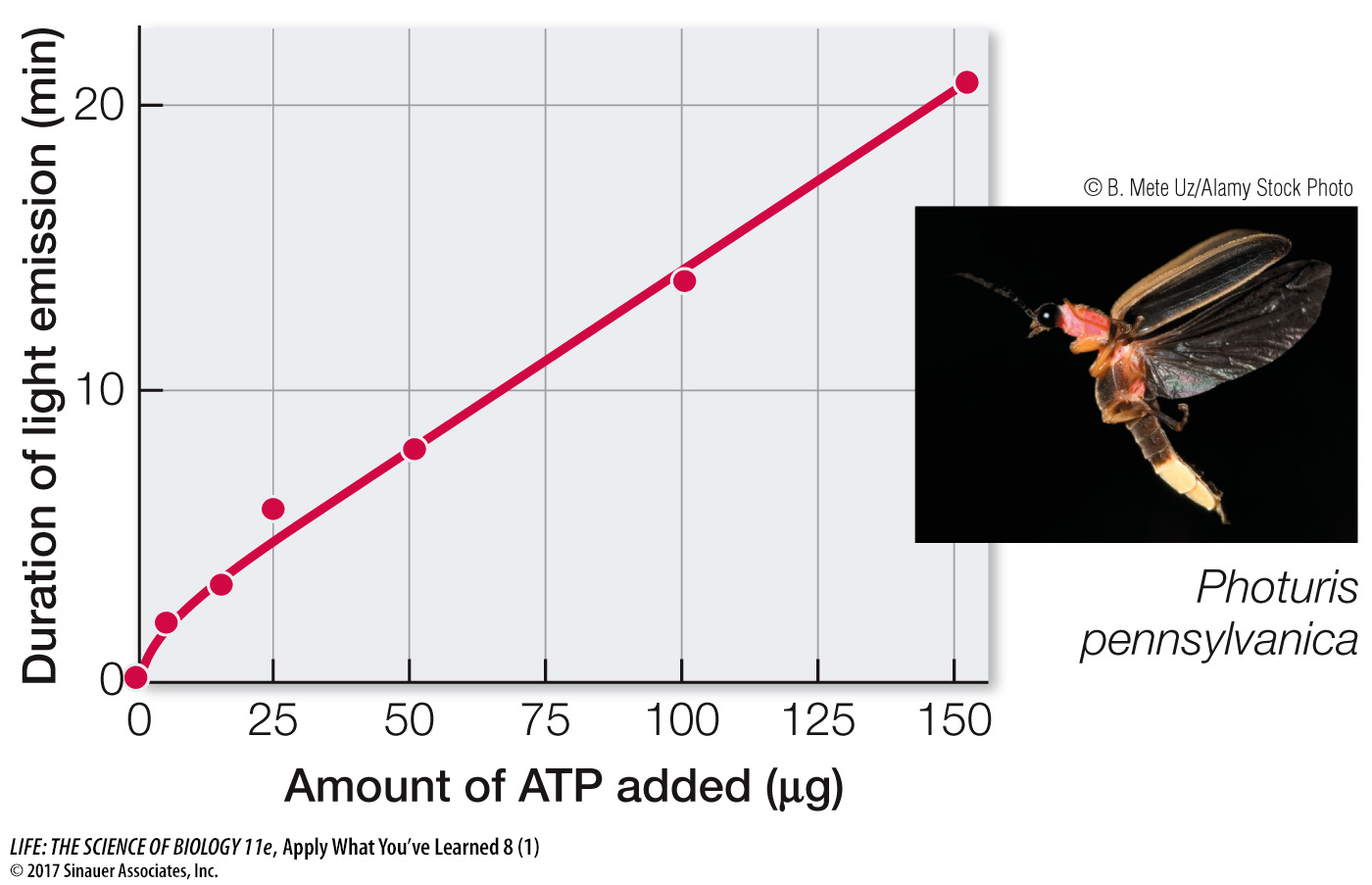
Through additional investigations, the researchers discovered that a compound called luciferin was responsible for the light emission as luciferin underwent a chemical reaction. The researchers purified an enzyme from extracts of firefly lanterns. The enzyme catalyzes the reaction in which luciferin emits light. In further studies, amounts of luciferin, ATP, and magnesium ion, Mg2+, were found to give the maximum light intensity (100%) when mixed with a fixed amount of enzyme. The table below shows how the relative light intensity was affected when variables were changed.
| Variable | Light intensity (%) |
|---|---|
| No enzyme | 0 |
| Enzyme heated before being added | 0 |
| No magnesium ion | 4 |
| 1 mM magnesium ion | 70 |
| 10 mM magnesium ion | 100 |
| pH 6.5 | 30 |
| pH 7.6 | 100 |
| pH 9.0 | 64 |
Questions
Question 1
Based on the information given, what set of molecules is necessary for the production of light by firefly lanterns, and what role does each molecule serve?
The molecules are luciferin, oxygen, ATP, and magnesium ion. Luciferin reacts with oxygen to undergo chemical change that involves emission of light as this change takes place. In order to drive this reaction, phosphate bonds in ATP are hydrolyzed, which releases chemical energy. Some of this energy is then released in the form of light as luciferin and oxygen react. Magnesium ion is needed as a cofactor of the enzyme that catalyzes reactions involving the other molecules.
Question 2
Explain how energy taken in by the firefly from its environment is made available for the production of light in a firefly lantern.
The firefly takes in food as a source of chemical energy. As the food is digested, some of the chemical energy in the bonds of the food molecules is stored in phosphate bonds of ATP as ADP is phosphorylated to form ATP. This chemical energy is released when ATP is hydrolyzed during the luciferin reaction, with some of the energy released in the form of light.
Question 3
Additional research has shown that an activated intermediate forms at the active site of the enzyme as luciferin reacts. This intermediate consists of luciferin covalently bonded to adenosine monophosphate (AMP). Still at the active site, this intermediate then reacts with oxygen to make an oxidized form of luciferin with the emission of light. The diagram below shows these steps. Redraw the diagram, adding steps to illustrate how coupling reactions could be involved to provide the energy needed for light emission.
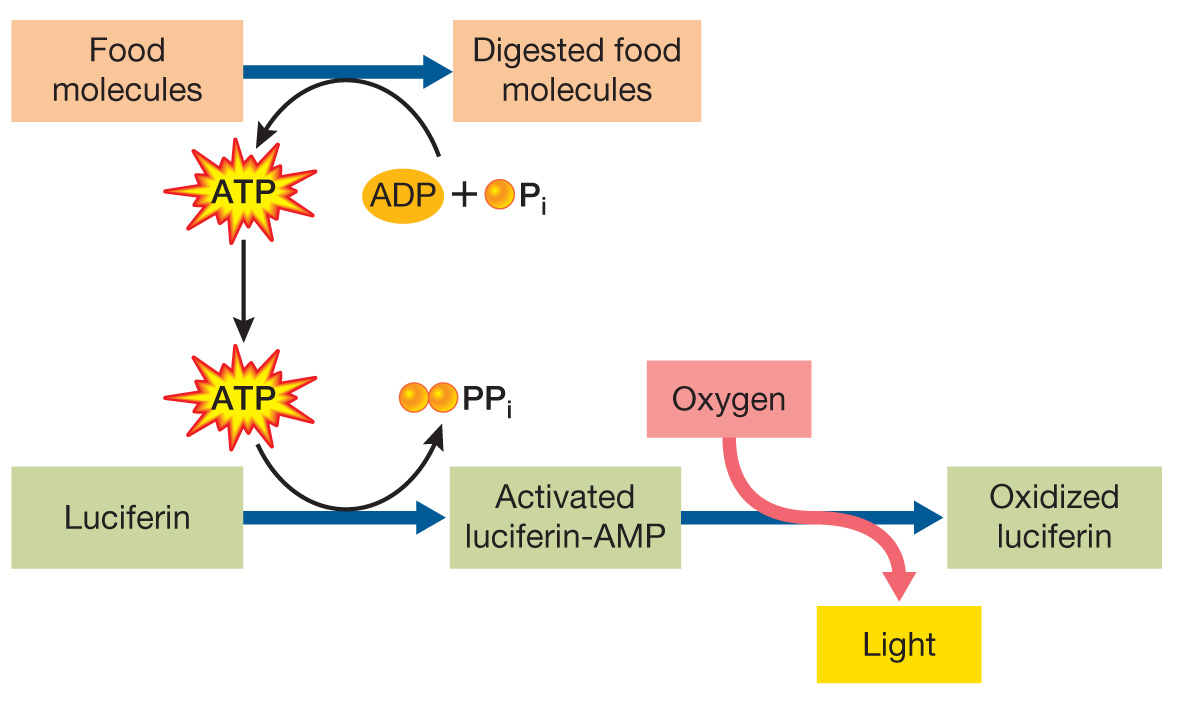
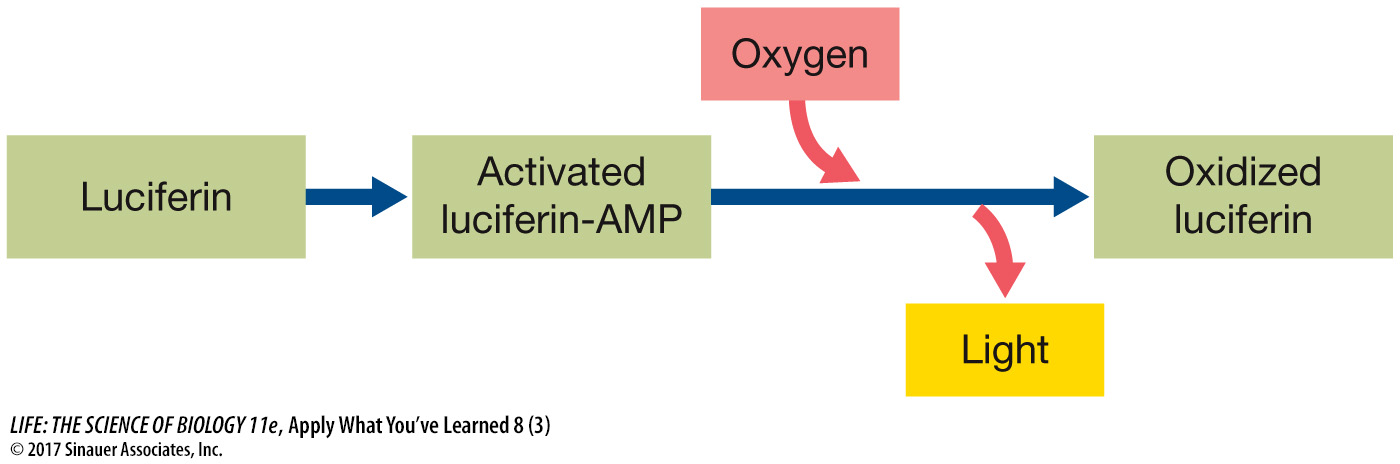
Question 4
What evidence is there that the enzyme is sensitive to environmental factors? Suggest possible reasons why this enzyme responds as it does to these factors.
The enzyme’s catalytic activity is abolished when the enzyme is heated. The catalytic activity is also affected by changes in pH. The effect of heating is likely due to disruption of the enzyme’s tertiary structure by high heat; most proteins cannot maintain their active shape if they are exposed to high heat. The effect of changing pH is likely due to changes in ionization of basic amino acid side chains of lysine and arginine or acidic side chains of aspartic acid and glutamic acid. Changes in ionization in these side chains can affect binding of the substrate or interactions between groups within the enzyme that stabilize its three-
Question 5
Some studies have shown that the enzyme undergoes a shape change upon binding ATP and luciferin. This change makes it impossible for a molecule of water to fit into the active site along with the substrates. What property of an enzyme does this represent, and how does this property help this particular enzyme carry out its role in catalysis?
This is an example of induced fit, which the enzyme uses to orient substrates with one another and with amino acid side chains of the enzyme that are important in its catalytic mechanism. This shape change of the enzyme excludes water from the active site, ensuring that ATP will not be hydrolyzed by water and will react only with luciferin to transfer AMP and release pyrophosphate, PPi.