Chemical reactions release or consume energy
As you saw earlier, anabolic reactions link simple molecules to form more complex molecules, so they tend to increase complexity (order) in the cell. By contrast, catabolic reactions break down complex molecules into simpler ones, so they tend to decrease complexity (generate disorder).
Catabolic reactions may break down an ordered reactant into smaller, more randomly distributed products. Reactions that release free energy (–ΔG) are called exergonic reactions (Figure 8.3A). For example:
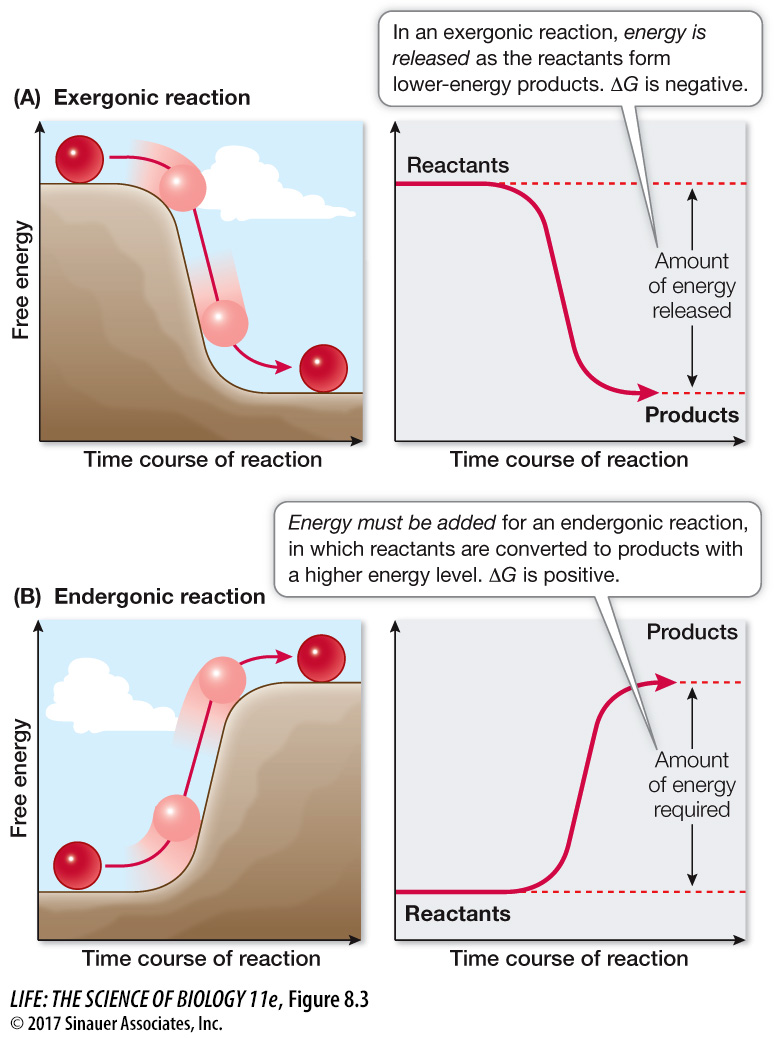 Figure 8.3 Exergonic and Endergonic Reactions (A) In an exergonic reaction, the reactants behave like a ball rolling down a hill, and energy is released. (B) A ball will not roll uphill by itself. Driving an endergonic reaction, like moving a ball uphill, requires the addition of free energy.
Figure 8.3 Exergonic and Endergonic Reactions (A) In an exergonic reaction, the reactants behave like a ball rolling down a hill, and energy is released. (B) A ball will not roll uphill by itself. Driving an endergonic reaction, like moving a ball uphill, requires the addition of free energy.Complex molecules → free energy + small molecules
Anabolic reactions may make a single product (a highly ordered substance) out of many smaller reactants (less ordered). Reactions that require or consume free energy (+ΔG) are called endergonic reactions (Figure 8.3B). For example:
Free energy + small molecules → complex molecules
In principle, chemical reactions are reversible and can run both forward and backward. For example, if compound A can be converted into compound B (A → B), then B, in principle, can be converted into A (B → A), although the concentrations of A and B determine which of these directions will be favored. You can think of the overall reaction as resulting from competition between the forward and reverse reactions (A ⇌ B). According to the law of mass action, increasing the concentration of A makes the forward reaction (A → B) happen more often than the reverse reaction, just as increasing the concentration of B favors the reverse reaction (B →A).
There are concentrations of A and B at which the forward and reverse reactions take place at the same rate. At these concentrations, no further net change in the system is observable, although individual molecules are still forming and breaking apart. This balance between forward and reverse reactions is known as chemical equilibrium. Chemical equilibrium is a state of no net change, and a state in which ΔG = 0.
154