The respiratory chain transfers electrons and protons, and releases energy
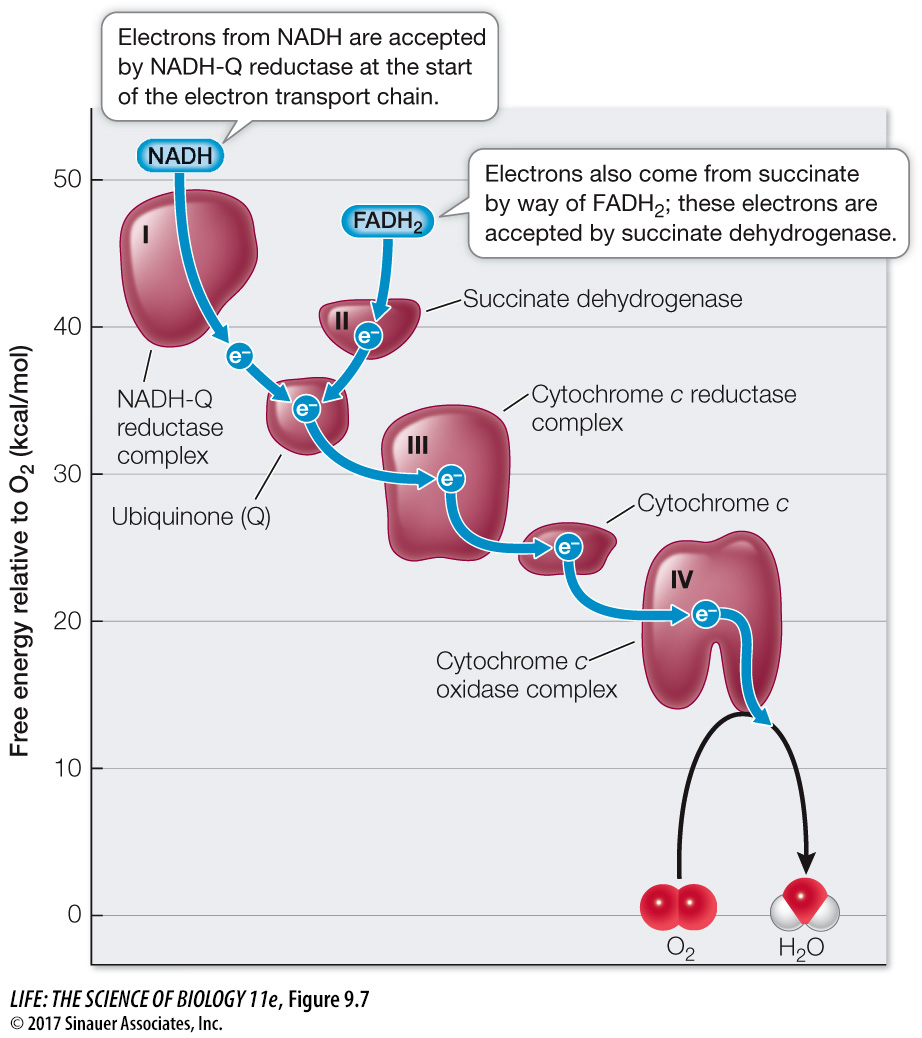
The respiratory chain is located in the inner mitochondrial membrane. Because of the extensive folding of the membrane, there is more room for the proteins involved in the chain than there would be in a membrane with less surface area. There are several interacting components, including large integral proteins, a small peripheral protein, and a small lipid molecule. Figure 9.7 shows a plot of the free energy released as electrons are passed between the carriers.
Question
Q: What is the ΔG for the transfer of electrons from cytochrome c to O2?
–20 kcal/mol
Activity 9.4 Respiratory Chain
www.life11e.com/
Four large protein complexes (I, II, III, and IV) contain electron carriers and associated enzymes. In eukaryotes they are integral proteins of the inner mitochondrial membrane (see Figure 5.11), and three are transmembrane proteins.
Cytochrome c is a small peripheral protein that lies in the intermembrane space. It is loosely attached to the outer surface of the inner mitochondrial membrane.
Ubiquinone (often referred to as coenzyme Q10; abbreviated Q) is a small, nonpolar, lipid molecule that moves freely within the hydrophobic interior of the phospholipid bilayer of the inner mitochondrial membrane.
As illustrated in Figure 9.7, NADH passes electrons to protein complex I (called NADH-
Complex III (cytochrome c reductase) receives electrons from Q and passes them to cytochrome c. Complex IV (cytochrome c oxidase) receives electrons from cytochrome c and passes them to oxygen. Finally the reduction of oxygen to H2O occurs:
O2 + 4 H+ + 4 e– → 2 H2O
Notice that four protons (H+) are also consumed in this reaction. This contributes to the proton concentration gradient across the inner mitochondrial membrane by reducing the proton concentration in the mitochondrial matrix.
180