Catabolism and anabolism are linked
A hamburger or veggie burger on a bun contains three major sources of carbon skeletons: carbohydrates, mostly in the form of starch (a polysaccharide); lipids, mostly as triglycerides (three fatty acids attached to glycerol); and proteins (polymers of amino acids). Look at Figure 9.13 to see how each of these three types of macromolecules can be hydrolyzed and used in catabolism or anabolism.
CATABOLIC INTERCONVERSIONS Polysaccharides, lipids, and proteins can all be broken down to provide energy:
Polysaccharides are hydrolyzed to glucose. Glucose then passes through glycolysis and cellular respiration, where its energy is captured in ATP.
Lipids are broken down into their constituents, glycerol and fatty acids. Glycerol is converted into dihydroxyacetone phosphate (DHAP), an intermediate in glycolysis. Fatty acids are highly reduced molecules that are converted to acetyl CoA inside the mitochondrion by a series of oxidation enzymes, in a process known as β-oxidation. For example, the β-oxidation of a 16-
carbon (C16) fatty acid occurs in several steps: C16 fatty acid + CoA → C16 fatty acyl CoA
C16 fatty acyl CoA + CoA → C14 fatty acyl CoA + acetyl CoA
Repeat 6 times → 8 acetyl CoA
The acetyl CoA can then enter the citric acid cycle and be catabolized to CO2.
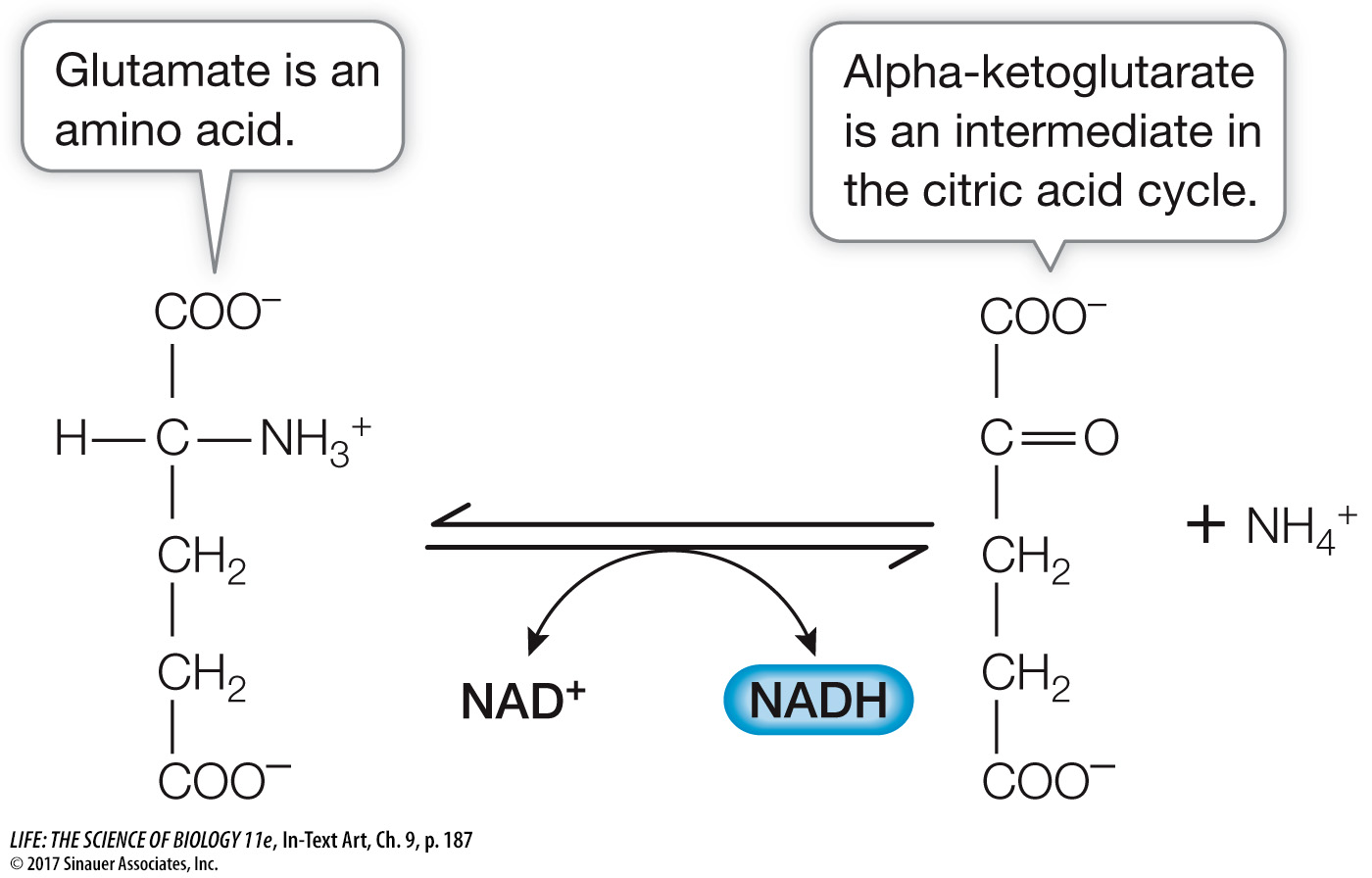
Proteins are hydrolyzed to their amino acid building blocks. The 20 different amino acids feed into glycolysis or the citric acid cycle at different points determined by their structures. For example, the amino acid glutamate is converted into α-ketoglutarate, an intermediate in the citric acid cycle (the five-
carbon molecule in Figure 9.6).
188
ANABOLIC INTERCONVERSIONS Many catabolic pathways can operate essentially in reverse, with some modifications. Glycolytic and citric acid cycle intermediates, instead of being oxidized to form CO2, can be reduced and used to form glucose in a process called gluconeogenesis (which means “new formation of glucose”). Likewise, acetyl CoA can be used to form fatty acids. The most common fatty acids have even numbers of carbons: 14, 16, or 18. These are formed by the addition of two-
Some intermediates in the citric acid cycle are reactants in pathways that synthesize important components of nucleic acids. For example, α-ketoglutarate is a starting point for purines, and oxaloacetate for pyrimidines. In addition, α-ketoglutarate is a starting point for the synthesis of chlorophyll (used in photosynthesis; see Chapter 10) and the amino acid glutamate (used in protein synthesis).