Metabolic pathways are regulated systems
The regulation of interconnecting biochemical pathways is a problem of systems biology, which seeks to understand how biochemical pathways interact (see Key Concept 8.5). It is a bit like trying to predict traffic patterns in a city: if an accident blocks traffic on a major road, drivers take alternate routes, where the traffic volume consequently changes.
Several mechanisms can be used to regulate the rate of each step in a biochemical pathway:
Changing the amount of active enzyme: The cell can increase the expression of a gene encoding an enzyme.
Changing enzyme activity by covalent modifications: Adding phosphate groups by protein kinase can alter the activity of an enzyme.
Feedback inhibition: Allosteric changes in an enzyme due to binding of a product in a pathway can cause the entire pathway to shut down.
Substrate availability: If the substrate of a particular enzyme is used up by another pathway, the first enzyme can no longer function and the pathway shuts down.
189
Consider what happens to the starch in your burger bun. In the digestive system, starch is hydrolyzed to glucose, which enters the blood for distribution to the rest of the body. But before the glucose is distributed, a regulatory check must be made: If there is already enough glucose in the blood to supply the body’s needs, the excess glucose is converted into glycogen and stored in the liver and muscles. If not enough glucose is supplied by food, glycogen is broken down, or other molecules are used to make glucose by gluconeogenesis. The end result is that the level of glucose in the blood is remarkably constant. How does the body accomplish this?
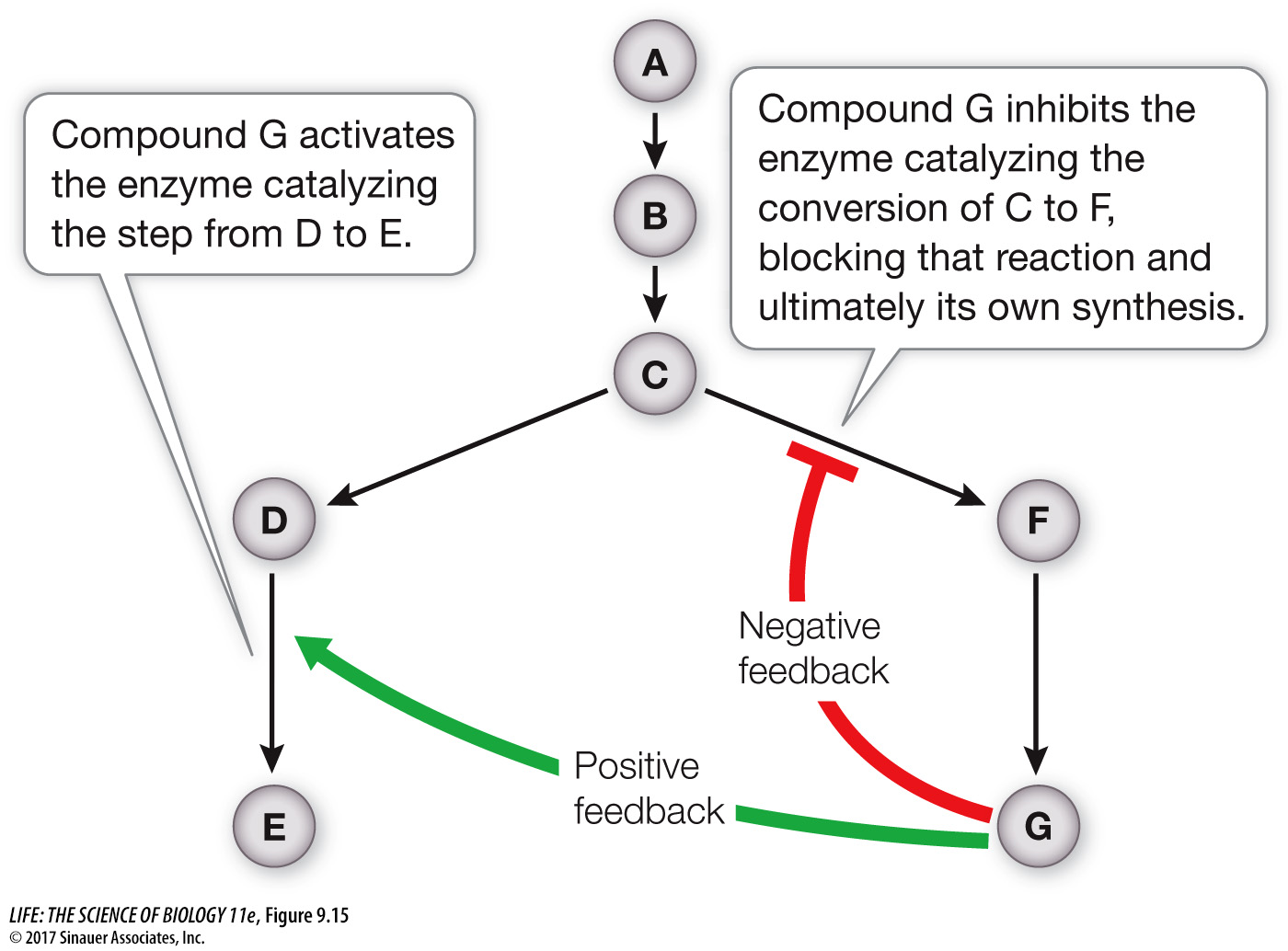
Glycolysis and the citric acid cycle are subject to allosteric regulation (see Key Concept 8.5) of key enzymes involved. In a metabolic pathway, a high concentration of the final product can inhibit the action of an enzyme that catalyzes an earlier reaction (see Figure 8.18). Furthermore, an excess of the product of one pathway can activate an enzyme in another pathway, speeding up its reactions and diverting raw materials away from synthesis of the first product (Figure 9.15). These negative and positive feedback mechanisms are used at many points in the energy-
Activity 9.7 Regulation of Energy Pathways
www.life11e.com/
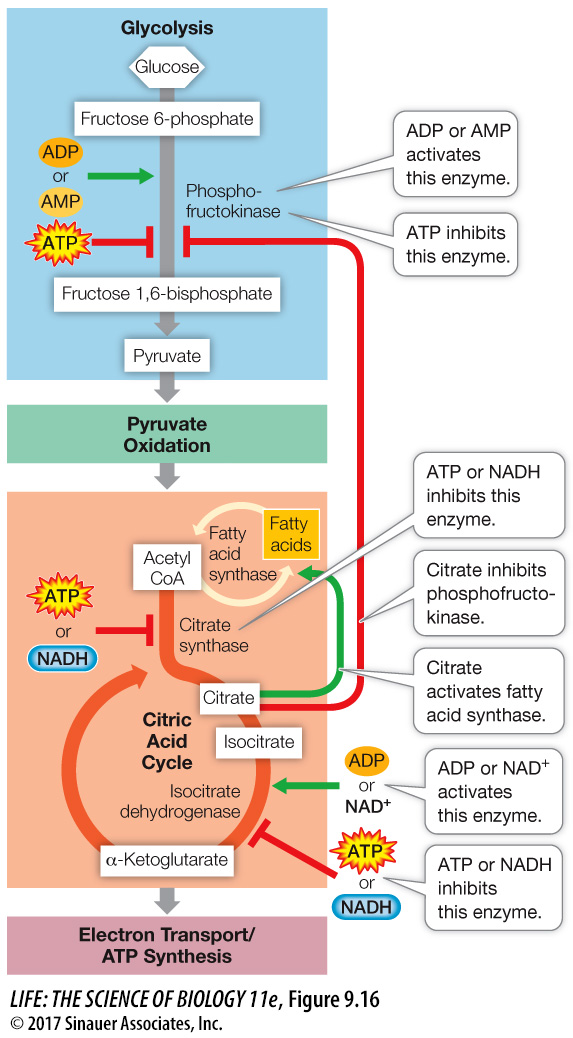
The main control point in glycolysis is the glycolytic enzyme phosphofructokinase, which catalyzes Step 3 in Figure 9.5. This enzyme is allosterically inhibited by ATP or citrate, and activated by ADP or AMP. Under anaerobic conditions, fermentation yields a relatively small amount of ATP, and phosphofructokinase operates at a high rate. However, when conditions are aerobic, respiration makes 16 times more ATP than fermentation does, and the abundant ATP allosterically inhibits phosphofructokinase. So glycolysis slows down.
The main control point in the citric acid cycle is the enzyme isocitrate dehydrogenase (which catalyzes Step 3 in Figure 9.6). This enzyme is activated by increases in substrate concentrations (ADP, NAD+, and isocitrate) and is inhibited by products of the citric acid cycle: ATP and NADH. If too much ATP or NADH accumulates, the reaction is slowed, and the citric acid cycle shuts down.
Another control point involves acetyl CoA. If the level of ATP is high and the citric acid cycle shuts down, the accumulation of citrate activates fatty acid synthase, diverting acetyl CoA to the synthesis of fatty acids for storage (contributing to a person’s accumulation of fat). These fatty acids may be metabolized later to produce more acetyl CoA.