How do cells obtain energy from glucose?
Cells obtain *energy from glucose by the chemical process of oxidation, which is carried out through a series of metabolic pathways. As you read this chapter, keep in mind five principles that govern metabolic pathways:
A complex chemical transformation occurs in a series of separate reactions that form a metabolic pathway.
Each reaction is catalyzed by a specific enzyme.
Many metabolic pathways are similar in all organisms, from bacteria to humans.
In eukaryotes, many metabolic pathways are compartmentalized, with certain reactions occurring inside specific organelles, or even specific regions of an organelle.
Some key enzymes in each metabolic pathway can be inhibited or activated to alter the rate of the pathway.
*connect the concepts The principles of energy transformations in living and nonliving systems are discussed in Key Concept 8.1.
As you saw in Key Concept 2.3, the familiar process of combustion (burning) is similar to the chemical processes that release energy in cells. If glucose is burned in a flame or is in a typical cell, it reacts with oxygen gas (O2), forming carbon dioxide and water and releasing energy in the form of heat. The balanced equation for the complete reaction is
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + free energy (ΔG = –686 kcal/mol)
This is an oxidation–
ADP + Pi + free energy → ATP
The oxidation of glucose when you heat it in the lab happens all at once. But in cells, glucose catabolism happens in many steps in a pathway. Each step is catalyzed by an enzyme, and the process is compartmentalized. Unlike combustion, glucose catabolism is tightly regulated and occurs at temperatures compatible with life.
Three catabolic processes harvest the energy in the chemical bonds of glucose: glycolysis, cellular respiration, and fermentation (Figure 9.1). All three processes involve pathways made up of many distinct chemical reactions.
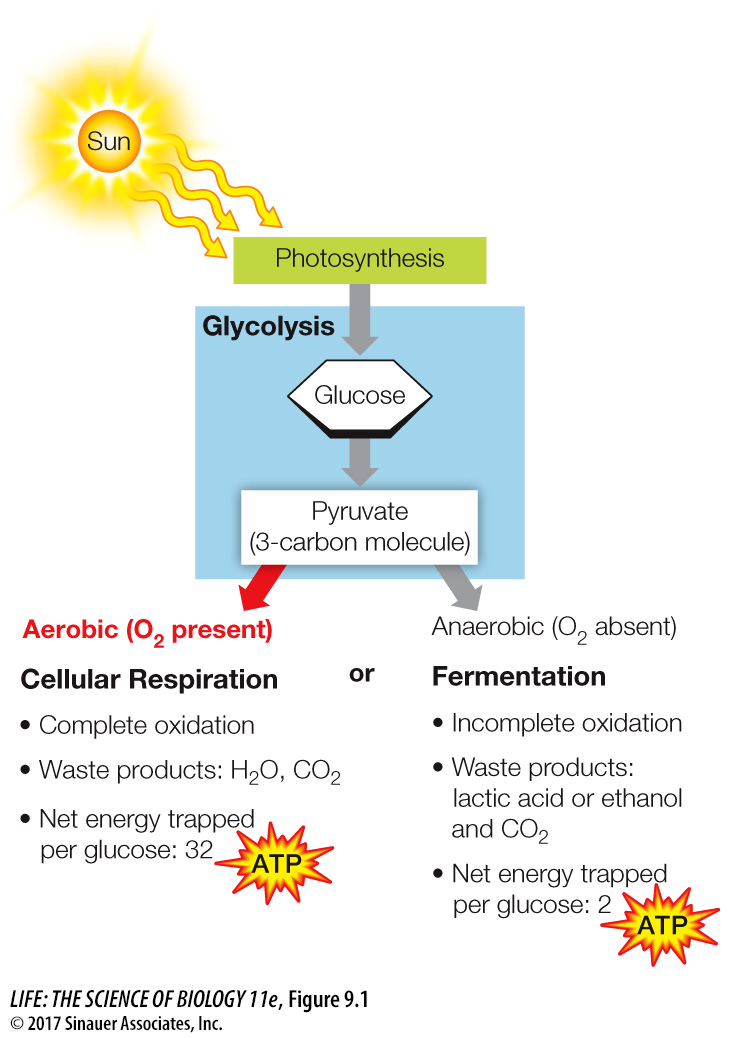
Glycolysis begins glucose catabolism. Through a series of chemical rearrangements, glucose is converted to two molecules of the three-
carbon product pyruvate, and a small amount of energy is captured in usable forms. Glycolysis is an anaerobic process because it does not require O2. Cellular respiration uses O2 from the environment and thus is aerobic. Each pyruvate molecule is completely converted into three molecules of CO2 through a set of catabolic pathways including pyruvate oxidation, the citric acid cycle, and an electron transport system (the respiratory chain). In the process, a great deal of the energy stored in the covalent bonds of pyruvate is captured to form ATP.
Fermentation does not involve O2 (it is anaerobic). With the exception of many microorganisms, fermentation converts pyruvate into lactic acid or ethyl alcohol (ethanol), both of which are still relatively energy-
rich molecules. Because the breakdown of glucose is incomplete, much less energy is released when glycolysis is coupled to fermentation than when it is coupled to cellular respiration.