Redox reactions transfer electrons and energy
174
As you saw in Key Concept 8.2, the addition of a phosphate group to ADP to make ATP is an endergonic reaction (see Figure 8.6). It is achieved by coupling an exergonic reaction to ATP production: the energy released in the exergonic reaction is used to drive ATP synthesis. Electrons are transferred in the exergonic reaction. A reaction in which one substance transfers one or more electrons to another substance is called an oxidation–
Reduction is the gain of one or more electrons by an atom, ion, or molecule.
Oxidation is the loss of one or more electrons.
Oxidation and reduction always occur together: as one chemical is oxidized, the electrons it loses are transferred to another chemical, reducing it. In a redox reaction, we call the reactant that becomes reduced an oxidizing agent and the one that becomes oxidized a reducing agent:
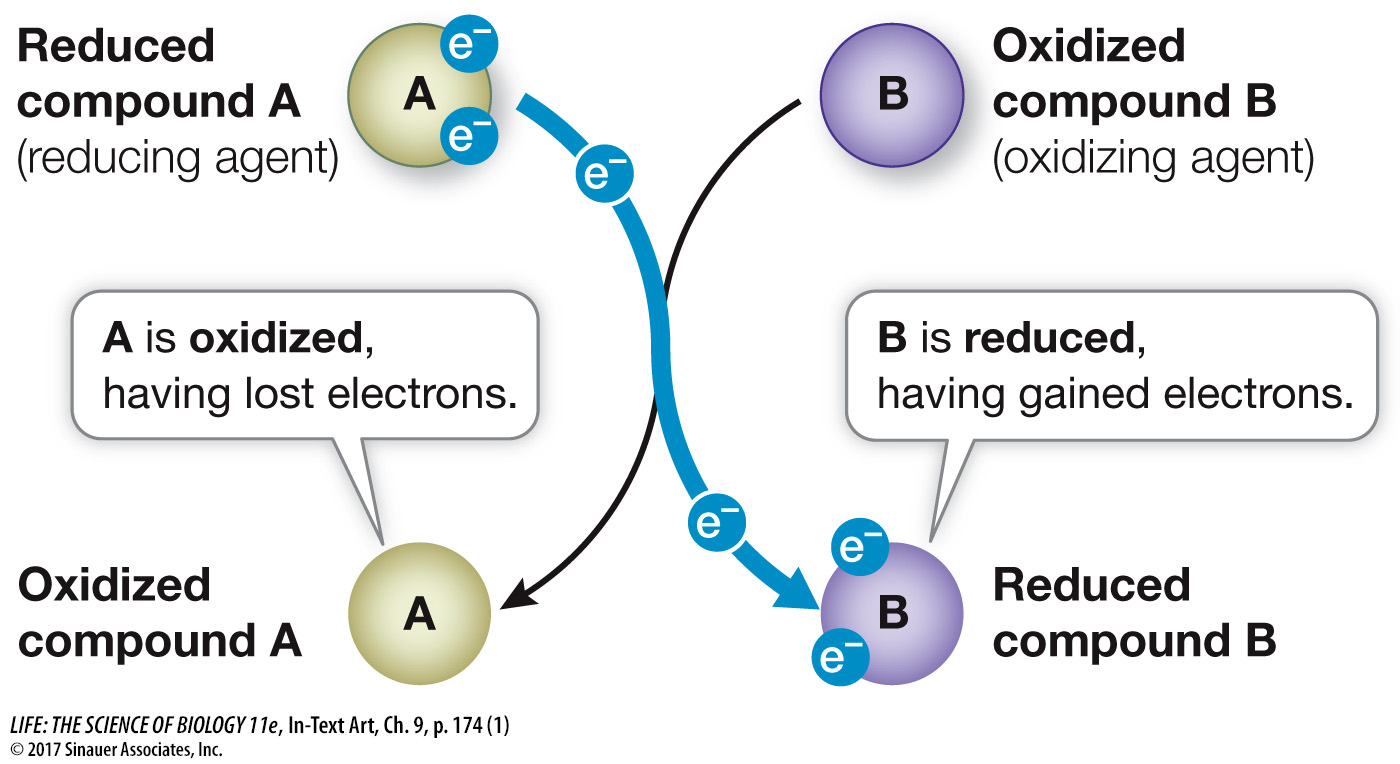
In the metabolism of glucose:
Glucose is the reducing agent (electron donor).
O2 is the oxidizing agent (electron acceptor).
Although oxidation and reduction are always defined in terms of electrons, it is often simpler to think in terms of the gain or loss of hydrogen atoms. The transfer of electrons is often associated with the transfer of hydrogen ions (a H atom contains H+ + e–). So when a molecule loses hydrogen atoms, it becomes oxidized.
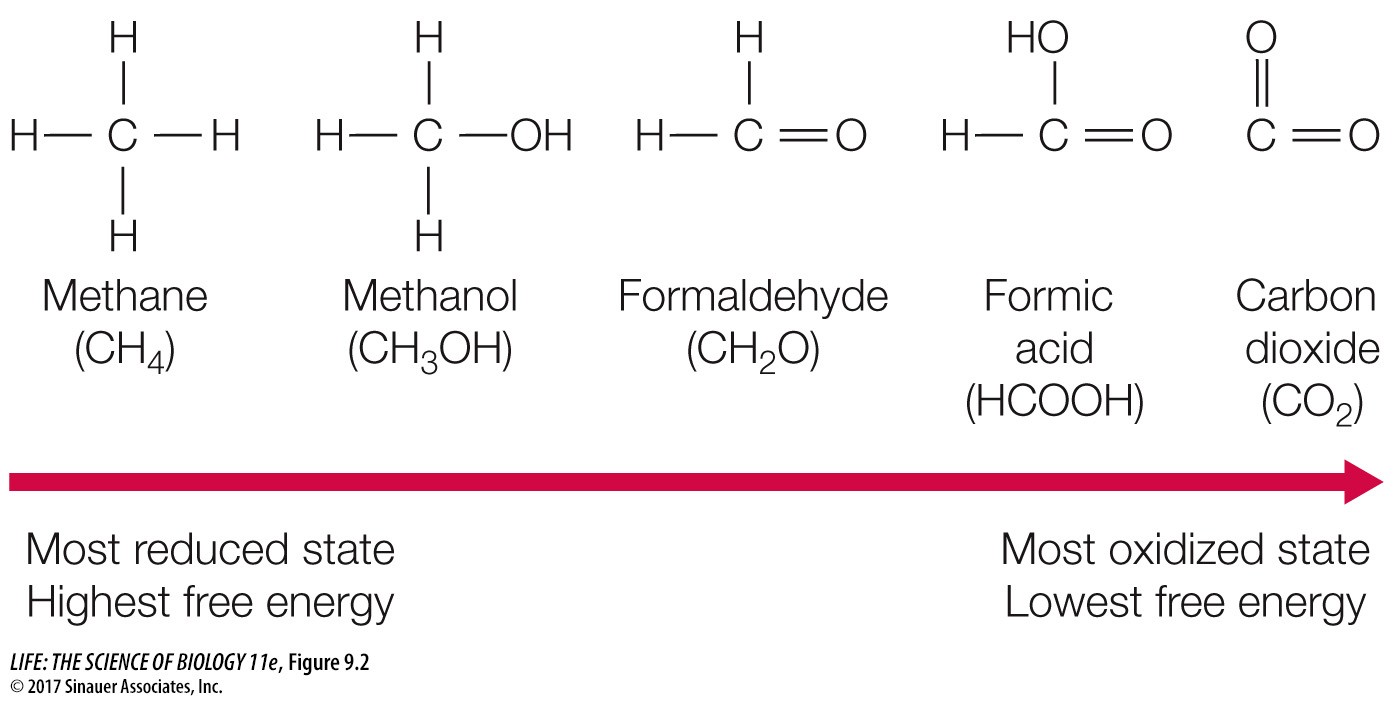
The more reduced a molecule is, the more energy is stored in its covalent bonds (Figure 9.2). In a redox reaction, some energy is transferred from the reducing agent to the reduced product. The rest remains in the reducing agent or is lost to entropy. As you will see, some of the key reactions of glycolysis and cellular respiration are highly exergonic redox reactions.