The coenzyme NAD+ is a key electron carrier in redox reactions
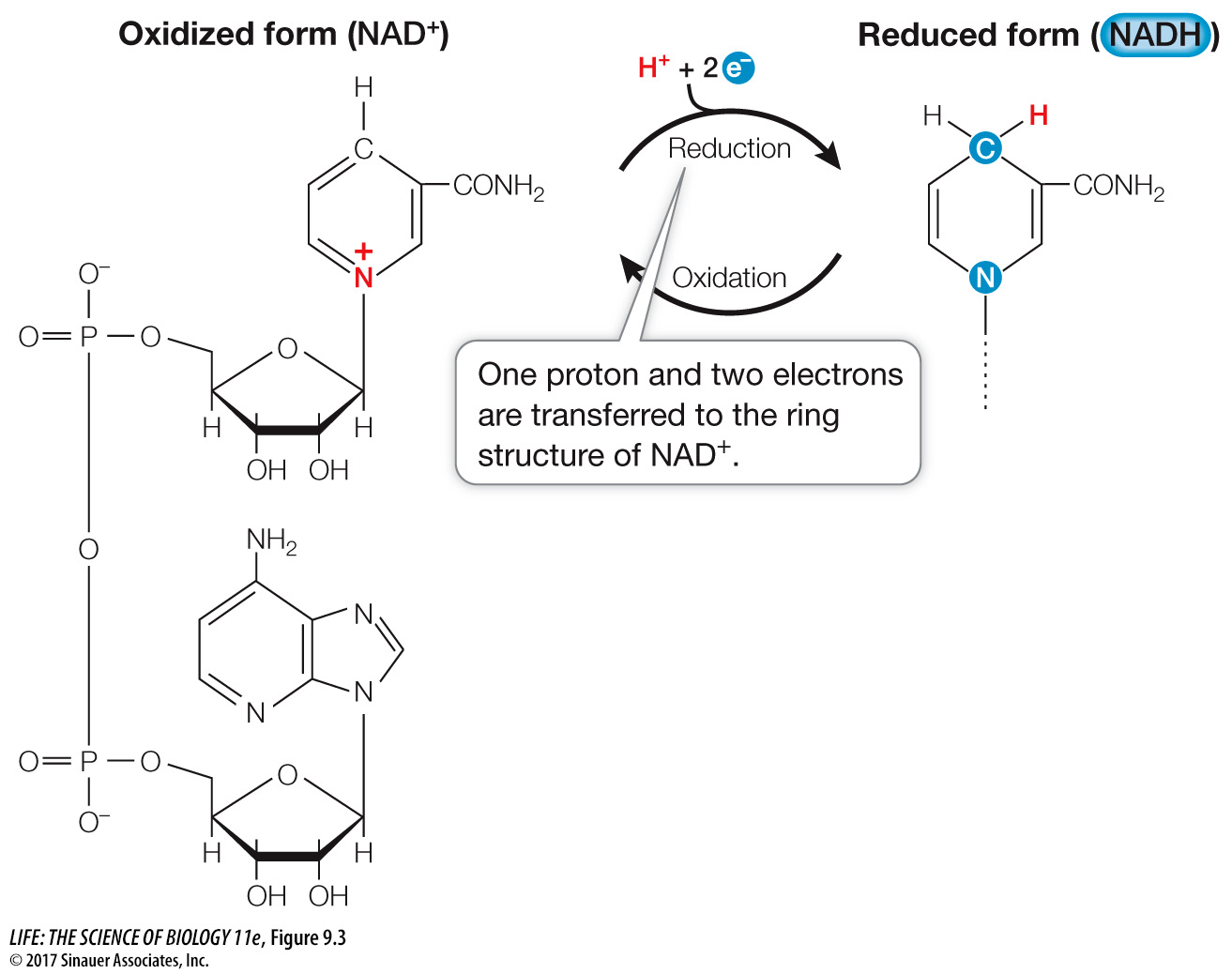
The *coenzyme nicotinamide adenine dinucleotide (NAD+) acts as an electron carrier in redox reactions. Note the flow of electrons along the blue lines below:

*connect the concepts Key Concept 8.4 describes the role of coenzymes, small molecules that assist in enzyme-
As you can see, NAD+ exists in two chemically distinct forms, one oxidized (NAD+) and the other reduced (NADH) (Focus: Key Figure 9.3). Both forms participate in redox reactions. The reduction reaction
NAD+ + H+ + 2 e– → NADH
is actually the transfer of a proton (the hydrogen ion, H+) and two electrons, which are released by the accompanying oxidization reaction.
The electrons do not remain with the coenzyme. Oxygen is highly electronegative and readily accepts electrons from NADH. The oxidation of NADH by O2 (which occurs in several steps)
NADH + H+ + ½ O2 → NAD+ + H2O
is exergonic, with a standard free energy change at pH 7 (ΔG°) of –52.4 kcal/mol (–219 kJ/mol). Note that the oxidizing agent appears here as “½ O2” instead of “O.” This notation emphasizes that it is molecular oxygen, O2, that acts as the oxidizing agent.
175
focus: key figure
Question
Q: Where does the “H” in red come from when NAD is reduced?
The H atom comes from the oxidation of a substrate.
Just as ATP can be thought of as a package of 7.3 kcal/mol (30.5 kJ/mol) of free energy, NADH can be thought of as a larger package of free energy (52.4 kcal/mol; see above). NAD+ is a common electron carrier in cells, but not the only one. Another carrier, flavin adenine dinucleotide (FAD), also transfers electrons during glucose metabolism.