Photosynthesis involves light and gas exchange
Plants, algae, and cyanobacteria live in aerobic environments and carry out oxygenic photosynthesis: the conversion of CO2 and water (H2O) into carbohydrates (which we will represent as a six-carbon sugar, C6H12O6) and oxygen gas (O2) (Figure 10.1).
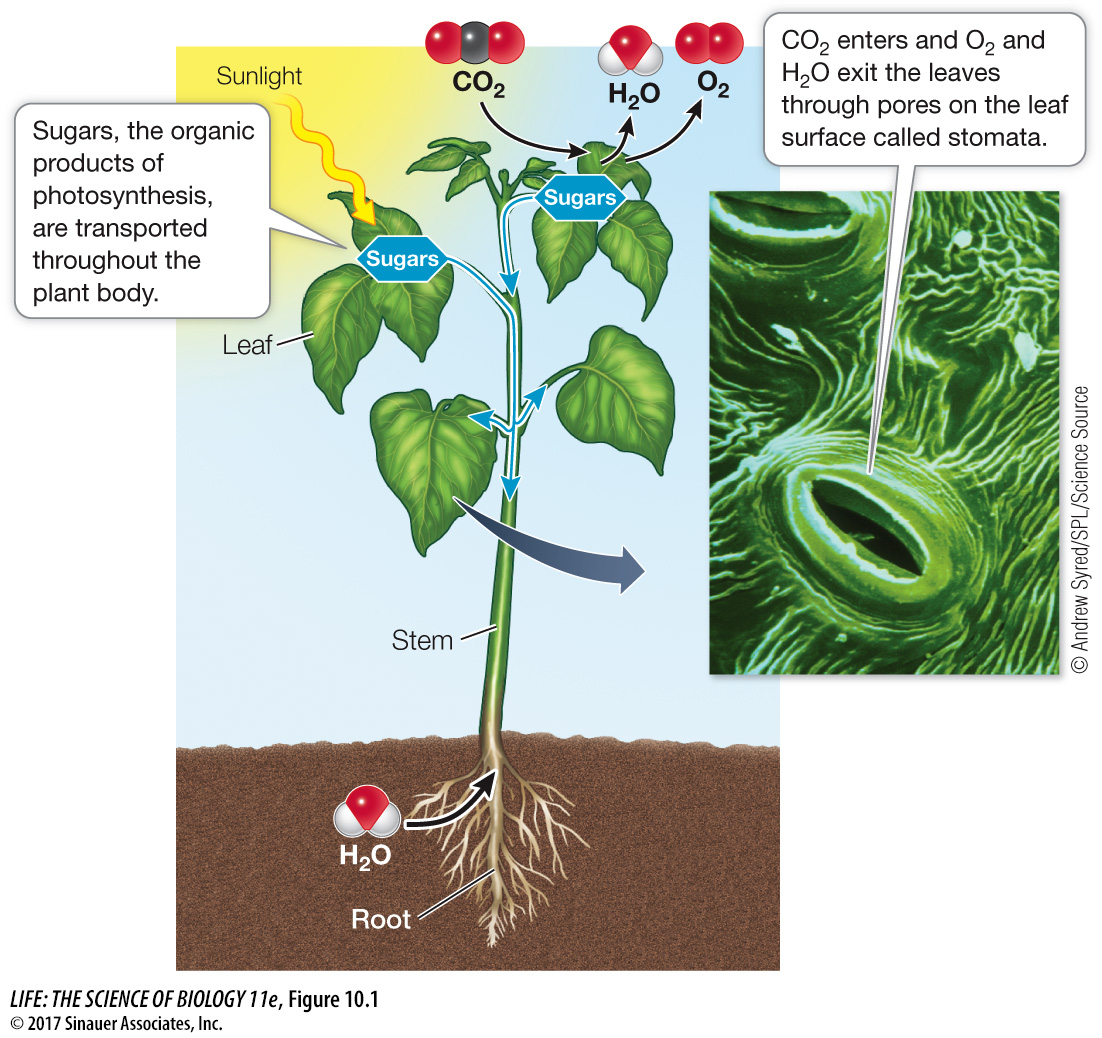
Figure 10.1 The Ingredients for Photosynthesis A typical terrestrial plant uses light from the sun, water from the soil, and carbon dioxide from the atmosphere to form organic compounds by photosynthesis.
6 CO2 + 6 H2O → C6H12O6 + 6 O2 (10.1)
Some kinds of bacteria live in anaerobic environments and carry out a type of photosynthesis in which energy from the sun is used to convert CO2 to more complex molecules without the production of O2. We will describe this process in more detail below, but first let’s look at oxygenic photosynthesis.
Equation 10.1 describes an endergonic reaction. From experiments such as the ones described in the chapter-opening story using FACE, the role of CO2 is well established. But while the equation is essentially correct, it is too general for a real understanding of the processes involved. Several questions arise: What are the precise chemical reactions of photosynthesis? What role does light play in these reactions? How do carbons become linked to form carbohydrates? What carbohydrates are formed? And does the oxygen gas come from CO2 or H2O?
