Experiments with isotopes show that O2 comes from H2O in oxygenic photosynthesis
In 1941 Samuel Ruben and Martin Kamen at the University of California, Berkeley performed experiments using the isotopes 18O and 16O to identify the source of the O2 produced during photosynthesis (Investigating Life: What Is the Chemistry of Photosynthesis, and How Will Increasing CO2 in the Atmosphere Affect It?). Their results showed that all the oxygen gas produced during photosynthesis comes from water, as is reflected in the revised balanced equation:
6 CO2 + 12 H2O → C6H12O6 + 6 O2 + 6 H2O (10.2)
Water appears on both sides of the equation because it is both used as a reactant (the 12 molecules on the left) and released as a product (the 6 new molecules on the right). This revised equation accounts for all the water molecules needed for all the oxygen gas produced.
The realization that water was the source of photosynthetic O2 led to an understanding of photosynthesis in terms of oxidation and reduction. As you learned in Chapter 9, oxidation–
12 H2O → 24 H+ + 24 e– + 6 O2 (10.3)
while carbon atoms in the oxidized state in CO2 get reduced to carbohydrate, with the simultaneous production of water:
6 CO2 + 24 H+ + 24 e–→ C6H12O6 + 6 H2O (10.4)
Adding Equations 10.3 and 10.4 (chemistry students will recognize them as half-
As you have just seen, water is the donor of protons and electrons in oxygenic photosynthesis. Earlier, we mentioned a type of photosynthesis that does not produce O2. In these cases, other molecules are used as electron donors in the reduction of CO2 to carbohydrates. For example, purple sulfur bacteria use hydrogen sulfide (H2S) as the electron donor:
12 H2S + 6 CO2 + light → C6H12O6 + 6 H2O + 12 S (10.5)
Green sulfur bacteria use sulfide ions, hydrogen, or ferrous iron as electron donors, whereas another group of bacteria use compounds derived from arsenic. The remainder of this chapter will focus on oxygenic photosynthesis, which produces the vast majority of the organic carbon used by life on Earth today and replenishes the O2 in our atmosphere.
195
investigating life
What Is the Chemistry of Photosynthesis, and How Will Increasing CO2 in the Atmosphere Affect It?
experiment
Original Paper: Ruben, S., M. Randall, M. D. Kamen and J. L. Hyde. 1941. Heavy oxygen (18O) as a tracer in the study of photosynthesis. Journal of the American Chemical Society 63(3): 877–
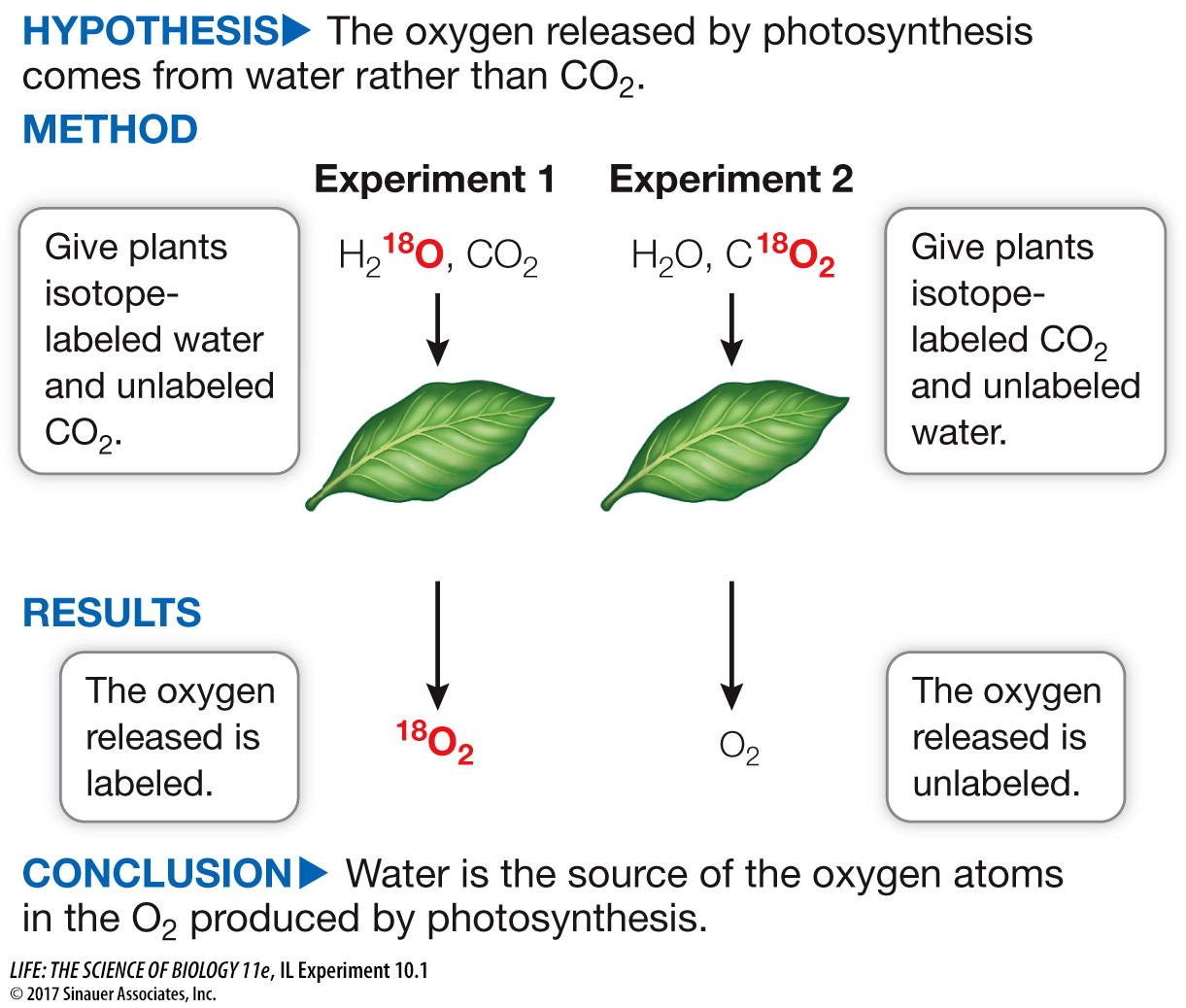
Understanding the chemical reactions of photosynthesis is key to understanding the effects of increasing atmospheric CO2. In particular, the source of O2 was not known. Two possibilities were the reactants, CO2 and H2O. In two separate experiments, Samuel Ruben and Martin Kamen labeled the oxygen in these molecules with the isotope 18O, then tested the O2 produced by a green plant to find out which molecules contributed the oxygen.
work with the data
In the 1930s, Cornelius van Niel, then a graduate student at Stanford University, was the first to propose that the oxygen released during photosynthesis is not actually derived from carbon dioxide, but rather from the water molecules consumed in the reaction. This hypothesis was formed on the basis of the discovery that the anaerobic purple sulfur bacteria do not release oxygen during photosynthesis. Instead, these organisms convert hydrogen sulfide (H2S) into elemental sulfur in their photosynthetic pathway (see Equation 10.5). This hypothesis was later confirmed by the experiment outlined above, which employed the “heavy” isotope of oxygen, 18O, to trace the flow of oxygen in plants.
As part of the expanding research on radioisotopes during World War II, the U.S. government set up a radiation laboratory at the University of California, Berkeley. Out of this lab came key experiments that described the light-
QUESTIONS
Question 1
In Experiment 1, was the isotopic ratio of O2 similar to that of H2O or to that of CO2? What about in Experiment 2?
In Experiment 1, the 18O/16O ratio of O2 (0.84–0.86) was similar to that of the H2O (0.85) and not to that of the CO2 sources (0.2–0.61). In Experiment 2, the ratio in O2 (0.20) was again more similar to that of the H2O (0.20) than to that of the CO2 sources (0.40–0.50).
Question 2
What can you conclude from these data?
The source of the oxygen atoms in O2 is H2O.
| 18O/16O (proportion 18O in compound) | |||||
|---|---|---|---|---|---|
| Time before start of O2 collection (min) |
Time at end of O2 collection (min) |
H2O | HCO3– + CO32– (CO2 sources) |
O2 | |
| Experiment 1: 0.09 M KHCO3 + 0.09 M K2CO3 (18O in H2O) |
0 | 0.85 | 0.20 | ||
| 45 | 110 | 0.85 | 0.41 | 0.84 | |
| 110 | 223 | 0.85 | 0.55 | 0.85 | |
| 225 | 350 | 0.85 | 0.61 | 0.86 | |
| Experiment 2: 0.14 M KHCO3 + 0.06 M K2CO3 (18O in CO2) |
0 | 0.20 | |||
| 40 | 110 | 0.20 | 0.50 | 0.20 | |
| 110 | 185 | 0.20 | 0.40 | 0.20 | |
Animation 10.1 The Source of the Oxygen Produced by Photosynthesis
www.life11e.com/
A similar work with the data exercise may be assigned in LaunchPad