Specific internal signals trigger events in the cell cycle
Cell fusion experiments were used to reveal the existence of internal signals that control the transitions between stages of the cell cycle. For example, an experiment involving the fusion of HeLa cells (the cells described in the opening story) at different phases of the cell cycle showed that a cell in S phase produces a substance that activates DNA replication (Investigating Life: What Controls the Reproduction of Cancer Cells?). Similar experiments pointed to the existence of signals controlling entry into M phase. Additional experiments showed that cell cycle progression signals are controlled by protein kinase activity.
217
investigating life
What Controls the Reproduction of Cancer Cells?
experiment
Original Paper: Rao, P. N. and R. T. Johnson. 1970. Mammalian cell fusion: Studies on the regulation of DNA synthesis and mitosis. Nature 225: 159–
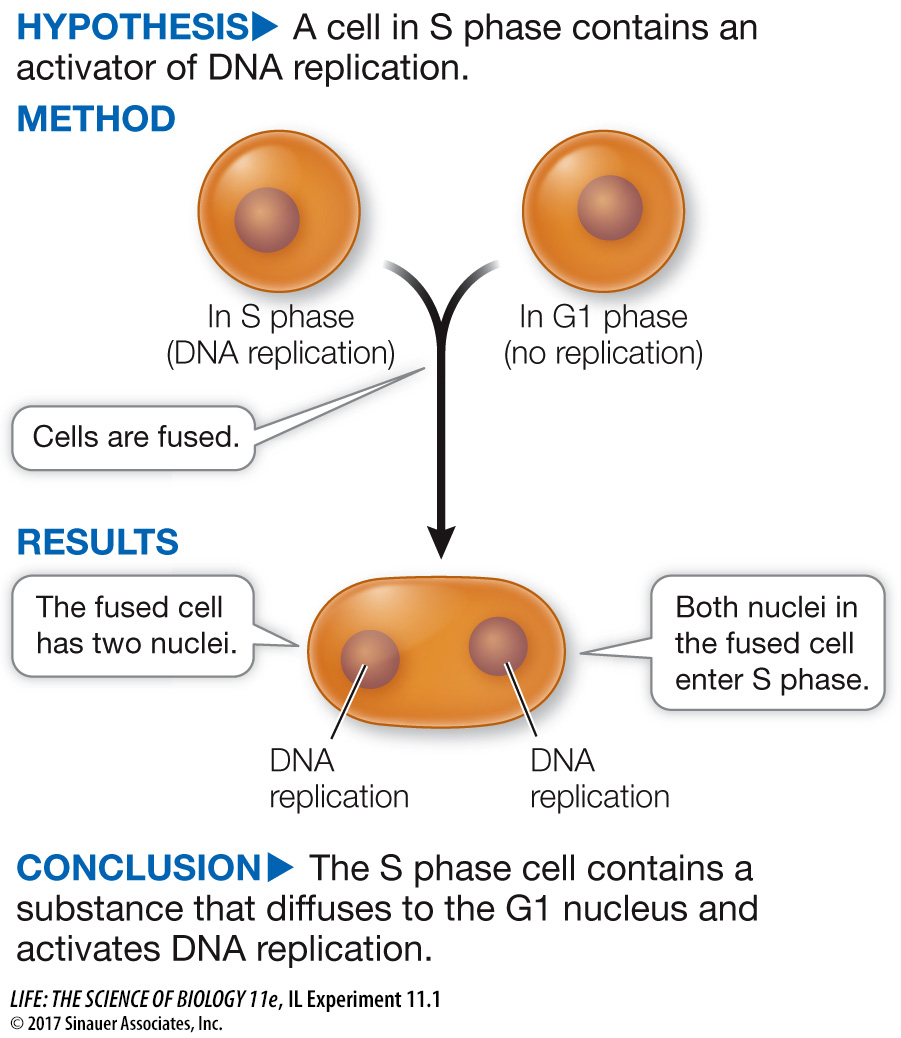
Nuclei of cells in G1 phase do not undergo DNA replication, but nuclei in S phase do. Rao and Johnson wondered whether substances present in cells in the S phase could be used to induce DNA replication in cells in the G1 phase.
work with the data
The fusion of cellular membranes is a natural process; it occurs during endocytosis and exocytosis, and in fertilization (the fusion of gametes). Membrane fusion also occurs when membrane-
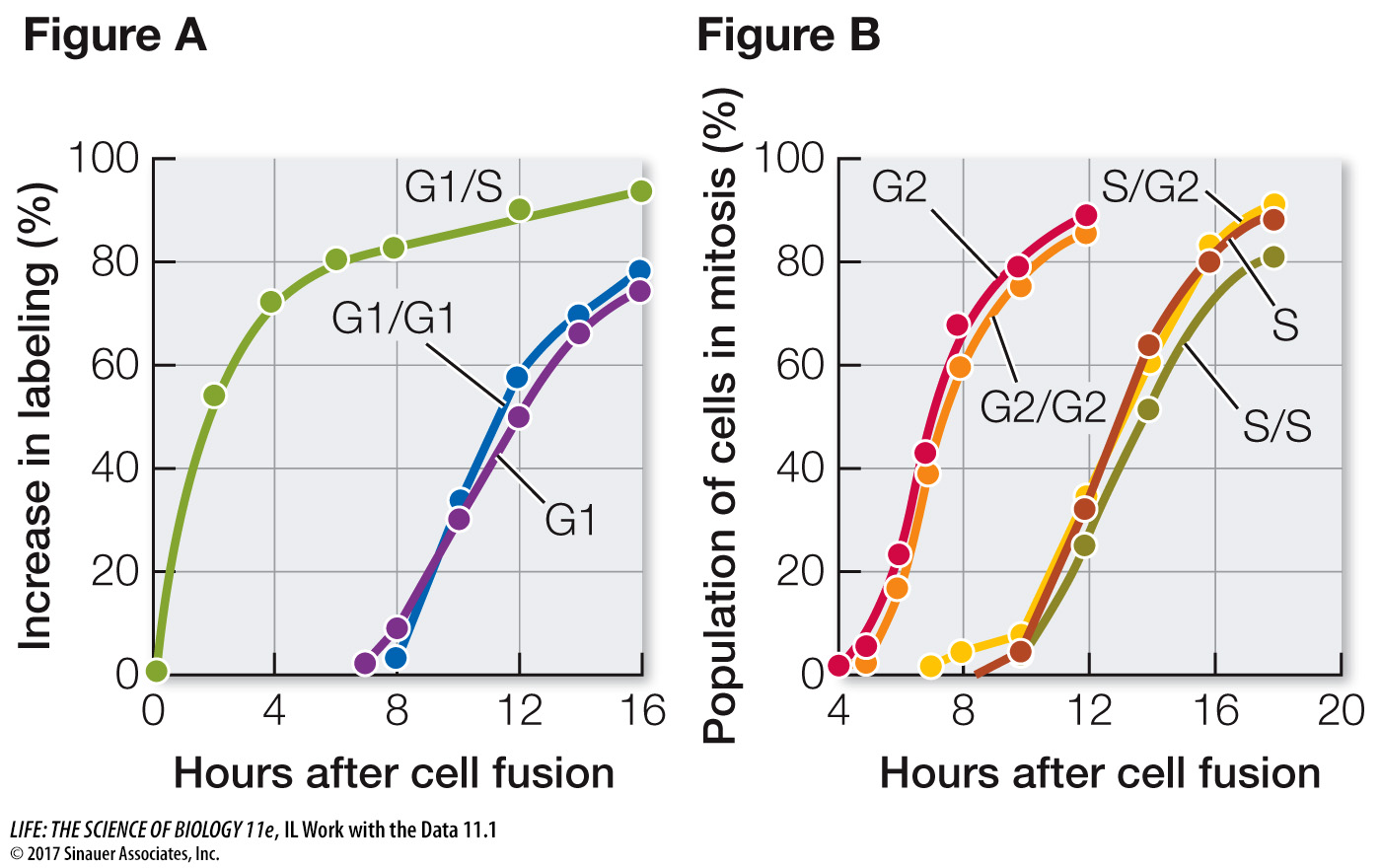
In their experiment, Rao and Johnson used HeLa cells, which divide continuously (see the opening story of this chapter). First, they isolated cells in either G1 or S phase. Before fusion, the cells in S phase were exposed to a radioactively labeled component of DNA (thymidine). The radioactivity was incorporated into these cells’ newly replicated DNA, labeling their nuclei. The S and G1 cells were then fused using Sendai virus (resulting in G1/S fusions) and again exposed to labeled thymidine. At various times after fusion, the scientists calculated the percent of previously unlabeled (G1) nuclei that had incorporated new label (i.e., had replicated their DNA) (Figure A). In a second series of experiments, S and G2 cells were fused in various combinations and then the numbers of cells in mitosis were counted and expressed as a percent of all cells in the population (Figure B).
QUESTIONS
Question 1
According to Figure A, how long did it take for all the G1 nuclei in the G1/S cells to become labeled?
Labeling of the G1 nuclei in the G1/S cells was mostly complete by 16 hours.
Question 2
Examine the data for fused G1/G1 cells and unfused G1 cells in Figure A. Explain why these were appropriate controls for the experiment. When did these nuclei become labeled? Compare these times with each other and with that for the G1/S nuclei and discuss.
The G1 control showed when DNA replication would normally occur in G1 nuclei. The G1/G1 control showed that the fusion process itself did not stimulate DNA replication; G1 cells had to be fused to S cells for DNA replication to be stimulated. Nuclei of the G1 and G1/G1 cells did not start to become labeled until about 8 hours after fusion, because these cells had to go all the way through G1 before entering S and replicating their DNA. By contrast, labeling of the G1 nuclei in the G1/S cells began soon after fusion.
Question 3
Examine the data in Figure B. Why did it take longer for the S phase cells to begin mitosis than the G2 cells?
G2 cells are further into the cell cycle than S phase cells are. It took several hours for the S phase cells to pass through S and G2 to begin mitosis.
Question 4
According to Figure B, did fusion with G2 cells alter the timing of mitosis in the S cell nuclei? Explain what this means in terms of regulation of the cell cycle.
The timing of mitosis in the hybrid S/G2 cells was similar to that in the unfused S cells and the S/S hybrids. This result indicates that G2 cells cannot stimulate mitosis of nuclei that are still in S phase.
Progress through the cell cycle depends on the activities of cyclin-
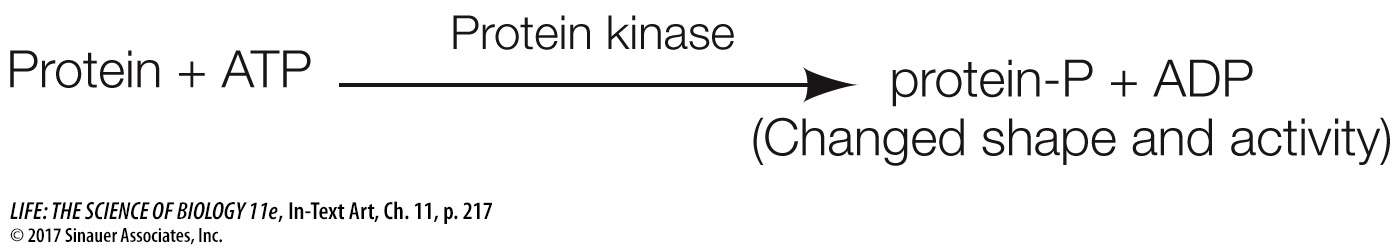
By catalyzing the phosphorylation of certain target proteins, Cdk’s play important roles at various points in the cell cycle. The discovery that Cdk’s induce cell division is a beautiful example of how research on different organisms and different cell types can converge on a single idea. One group of scientists, led by James Maller at the University of Colorado, was studying immature frog eggs, trying to find out how they are stimulated to divide and eventually form mature eggs. They found that when they added a protein from maturing eggs to immature eggs cells, the latter were stimulated to divide. They named the protein, maturation promoting factor.
Meanwhile, Leland Hartwell at the University of Washington was studying the cell cycle in yeast (a single-
Similar Cdk’s were soon found to control the G1-
218
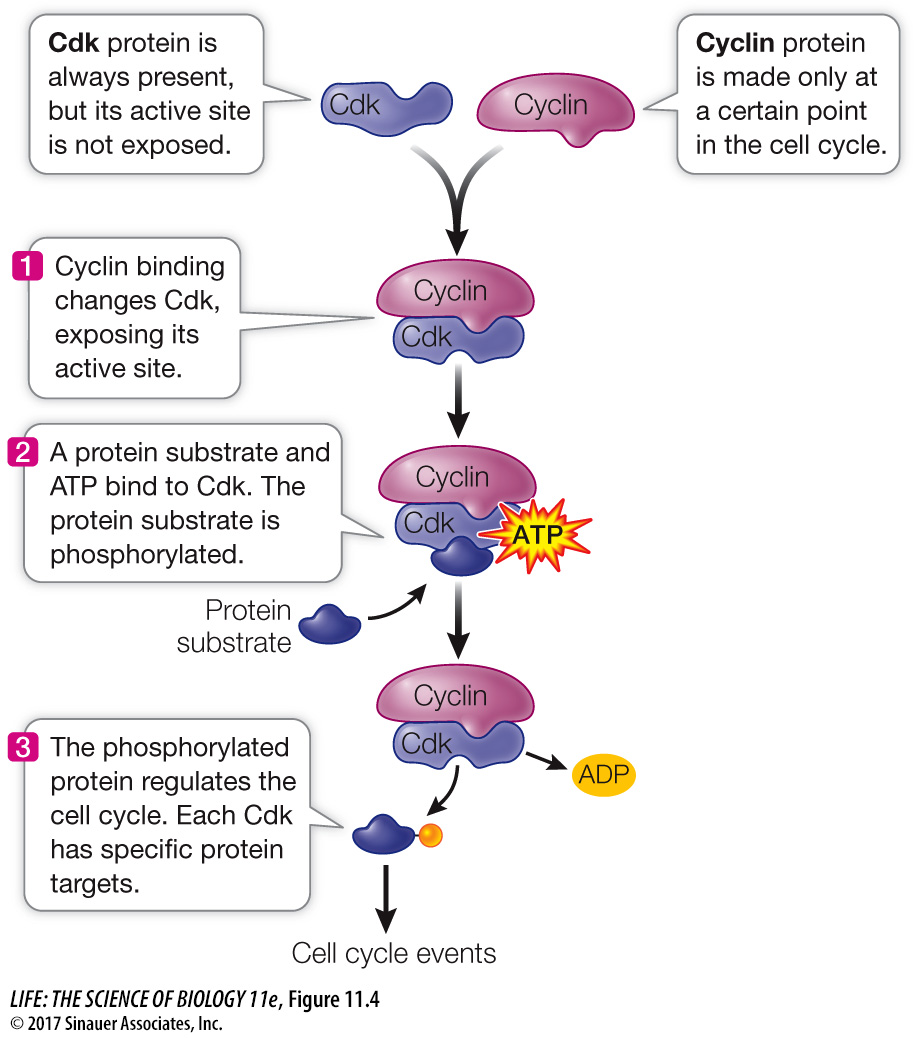
Cdk’s are not enzymatically active as protein kinases unless they are bound to another class of protein, the activator called cyclin. This binding—
*connect the concepts As discussed in Key Concept 8.5, allosteric regulation occurs when another molecule induces a change in the three-
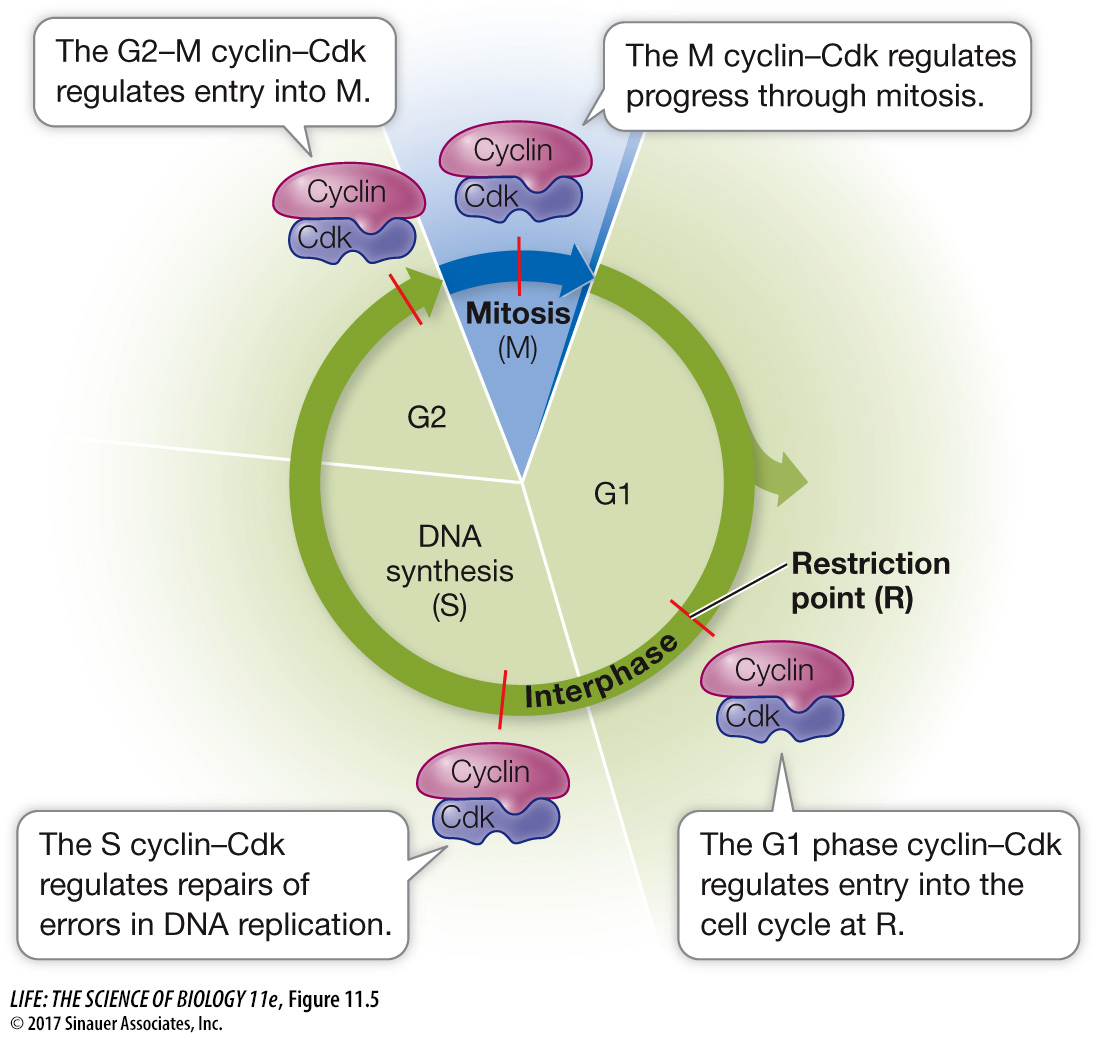
Cyclin–
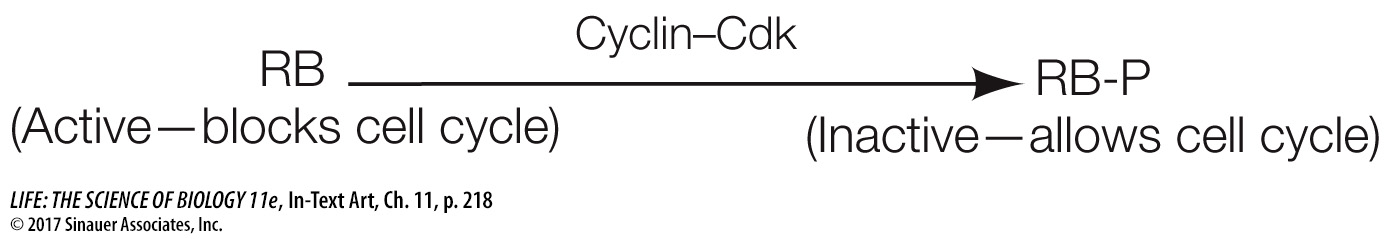
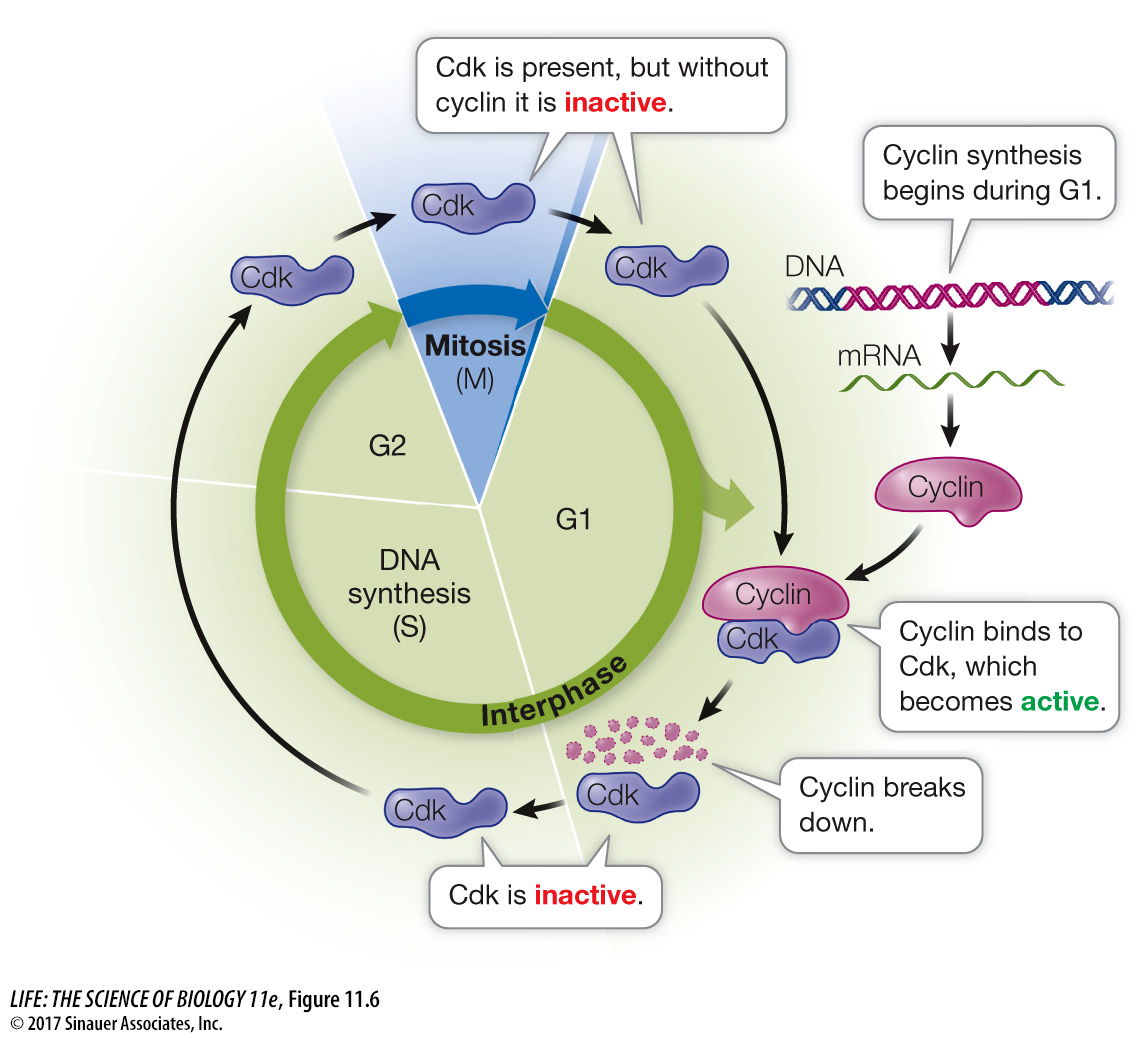
Progress through the cell cycle is regulated by the activities of Cdk’s, and so regulating them is a key to regulating cell division. An effective way to regulate Cdk’s is to regulate the presence or absence of cyclins (Figure 11.6). Simply put, if a cyclin is not present, its partner Cdk is not active. As their name suggests, cyclins are present cyclically: they are made only at certain times in the cell cycle.
The different cyclin–
| Cell cycle phase | Checkpoint trigger |
|---|---|
| G1 | DNA damage |
| S | Incomplete replication or DNA damage |
| G2 | DNA damage |
| M | Chromosome unattached to spindle |
As an example, let’s consider the G1 checkpoint (R). If DNA is damaged by radiation during G1, a signaling pathway results in the production of a protein called p21. (The p stands for “protein” and the 21 stands for its molecular weight—
219